
Alphabetical Index
Chemical Composition of Steels
Keyword Search
Steel Names
Alloyed Steels
Carbon Steels
Cast Irons
Chromium Steels
Cold Work Tool Steels
Creep Resistant Steels
Hot Work Tool Steels
Molybdenum Steels
PM steels
Stainless Steels
Structural Steels
Tool Steels
Vanadium Steels
White Cast Irons
M2C Carbides
M3C Carbides
M7C3 Carbides
M23C6 Carbides
MC Carbides
Light Microscopy
EDS/WDS Microanalysis
Scanning Electron Microscopy
Transmission Electron Microscopy
X-Ray Diffraction
Help
Contact Us
Home
Precipitates in austenitic steels
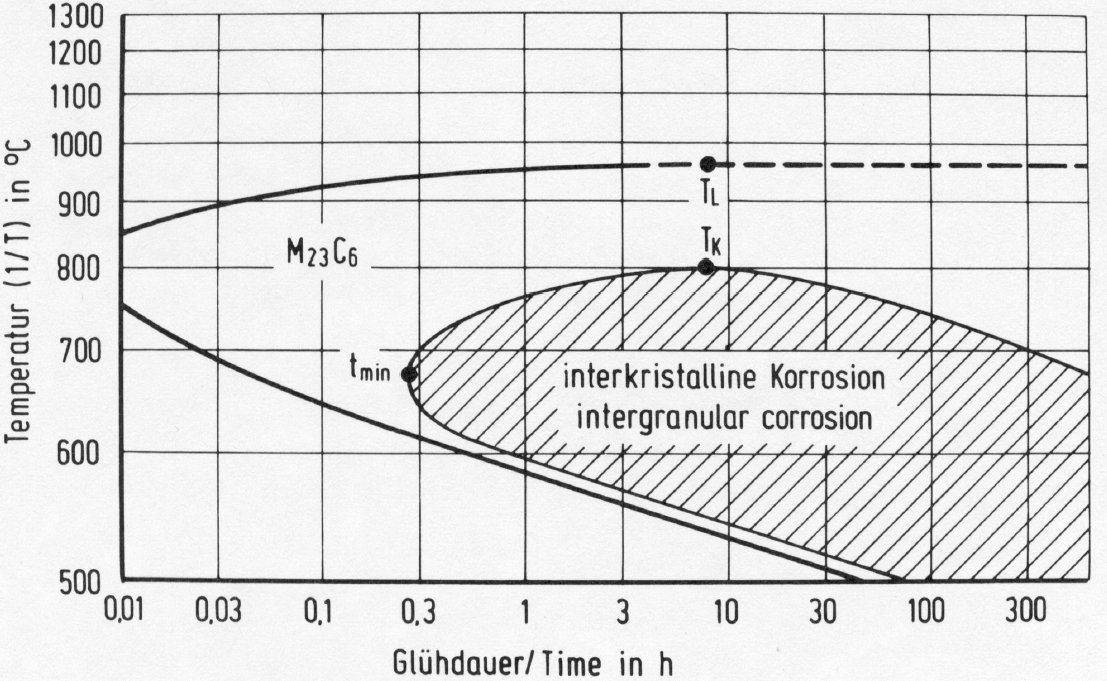
Figure 22: Time-temperature-precipitation diagram of the steel X5CrNi18-9.
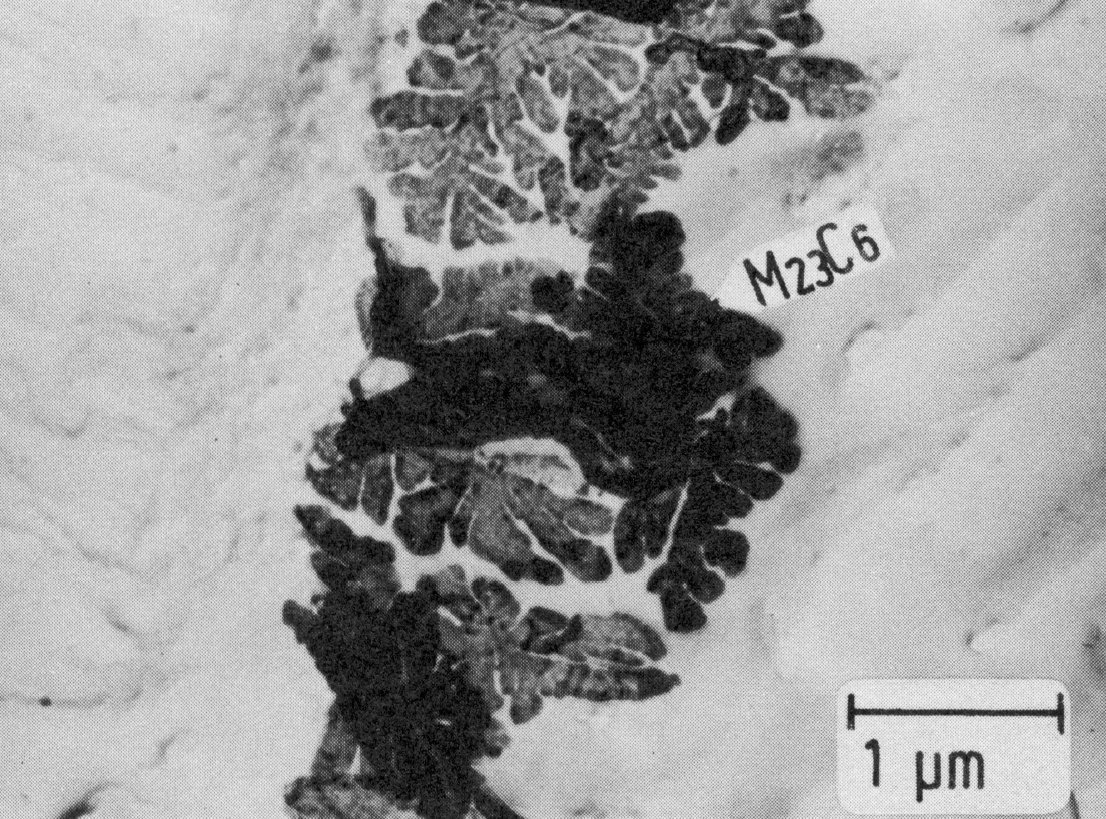
Figure 23: 20% Cr, 5% Ni, 5% Mn, 5% Al 1100 °C 1 h/W, extraction replica. Scale bar: 1 µm.
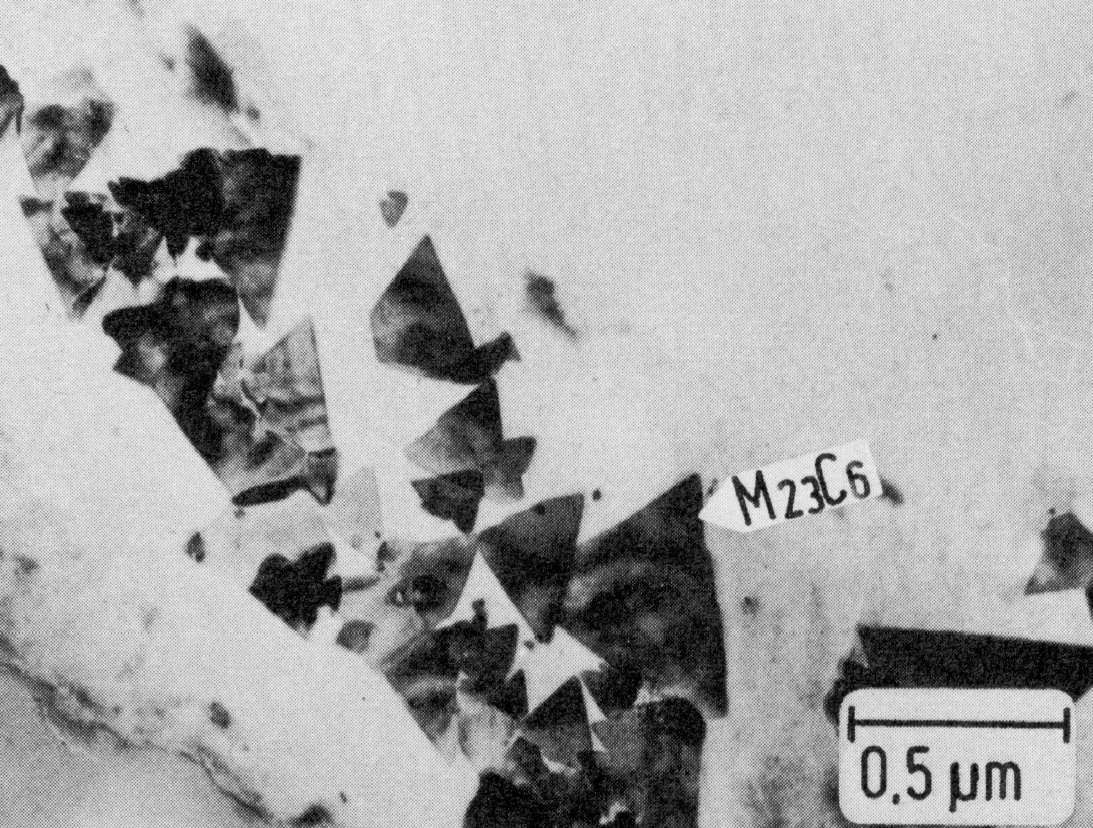
Figure 24: 16.6% Cr, 16.6% Ni, 2.2% Mo, 1100 °C 1 h/W + 600 °C 100 h/air, extraction replica. Scale bar: 0.5 µm.
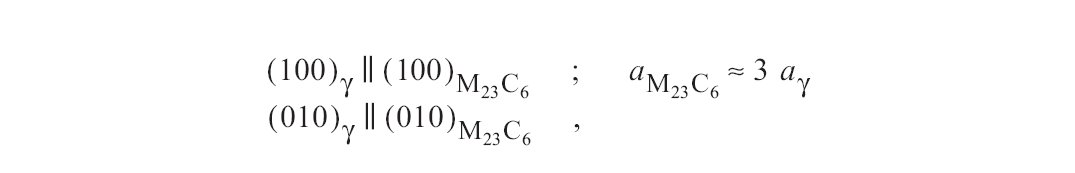
Figure a: M23C6 often precipitates with coherent nucleation in the austenite. The orientation relationship is (Fig. 26).
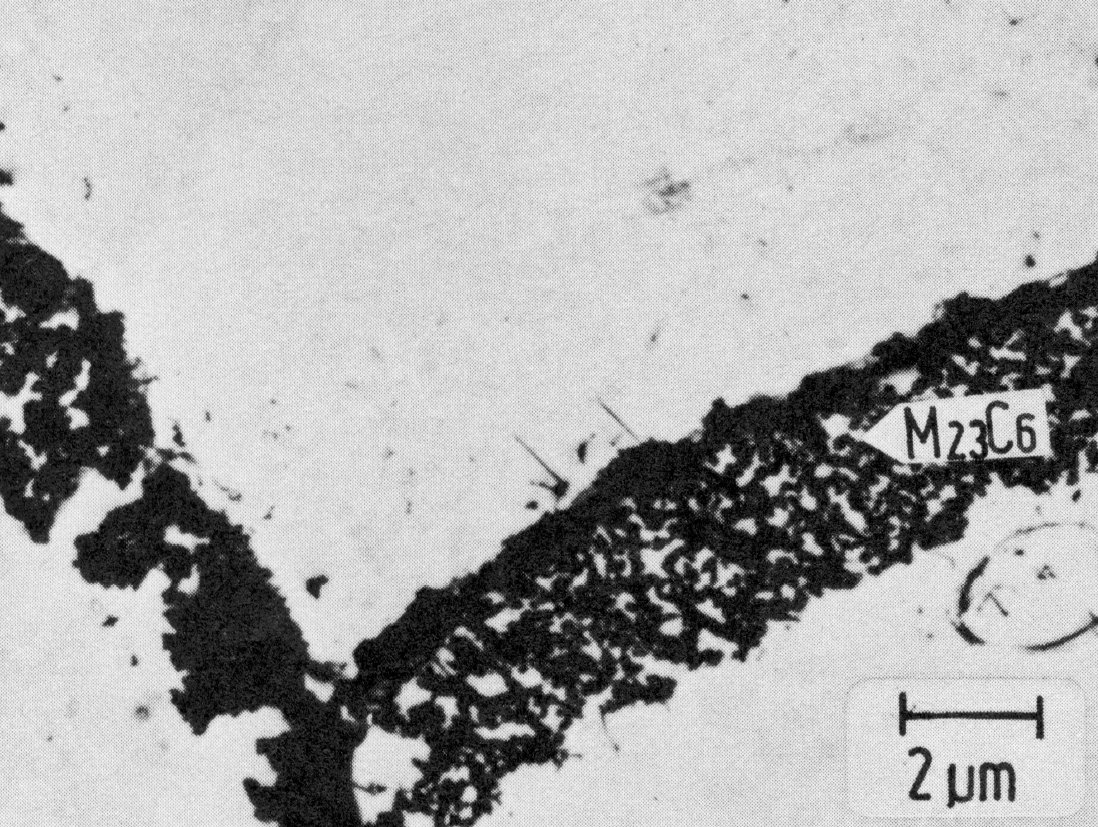
Figure 25: 16.7% Cr, 12.6% Ni, 4.5% Mo, 1100 °C 1 h/W + 600 °C 1000 h/air, extraction replica. Scale bar: 2 µm.

Figure 26: Orientation relationship between austenite and M23C6 electron diffraction pattern.
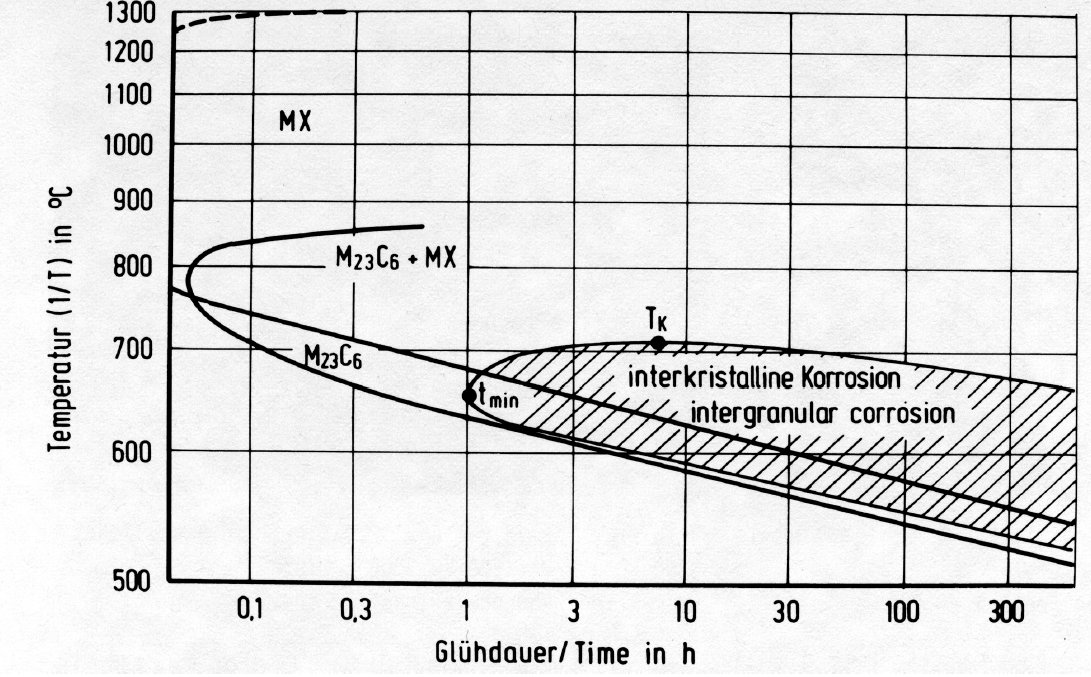
Figure 27: Time-temperature precipitation diagram of the steel X10CrNiNb18-9.
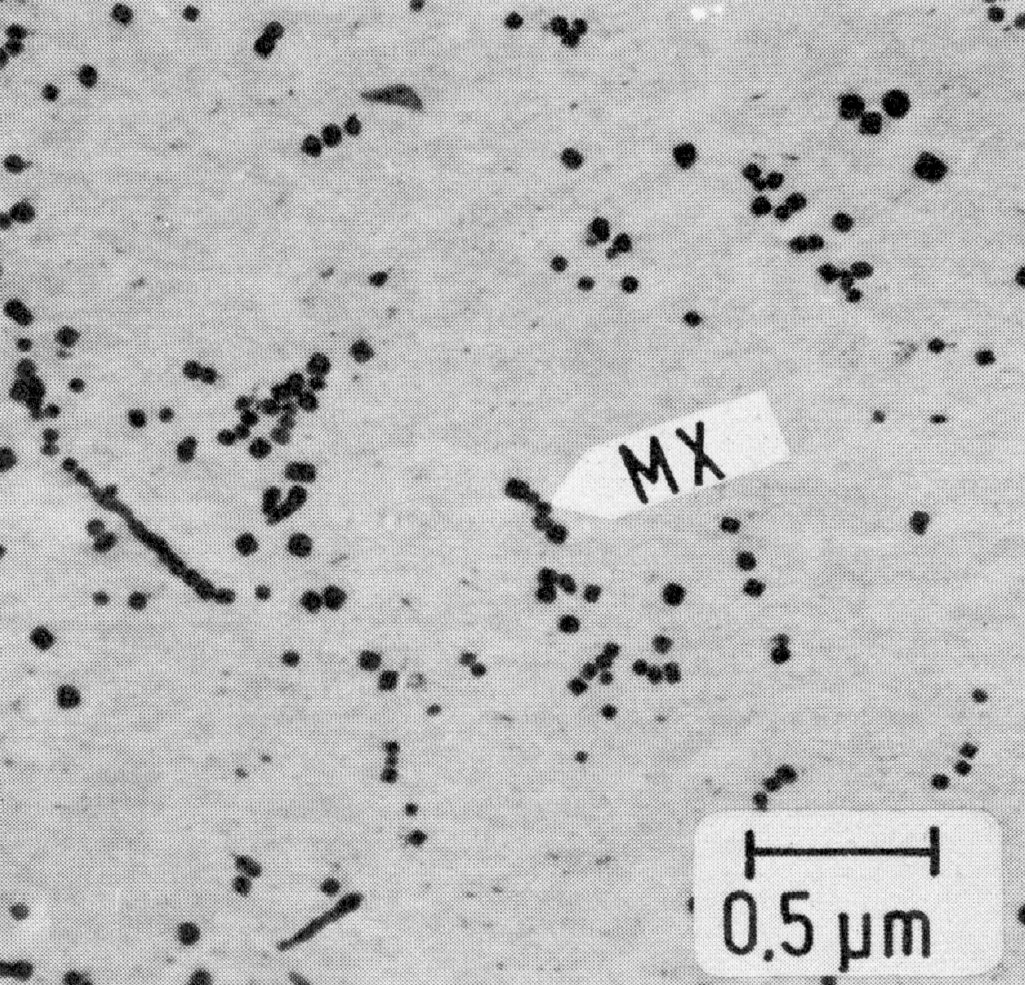
Figure 28: X10CrNiNb18-9, 1300 °C 15 min/W + 1200 °C 3 h/W, extraction replica. Scale bar: 0.5 µm.
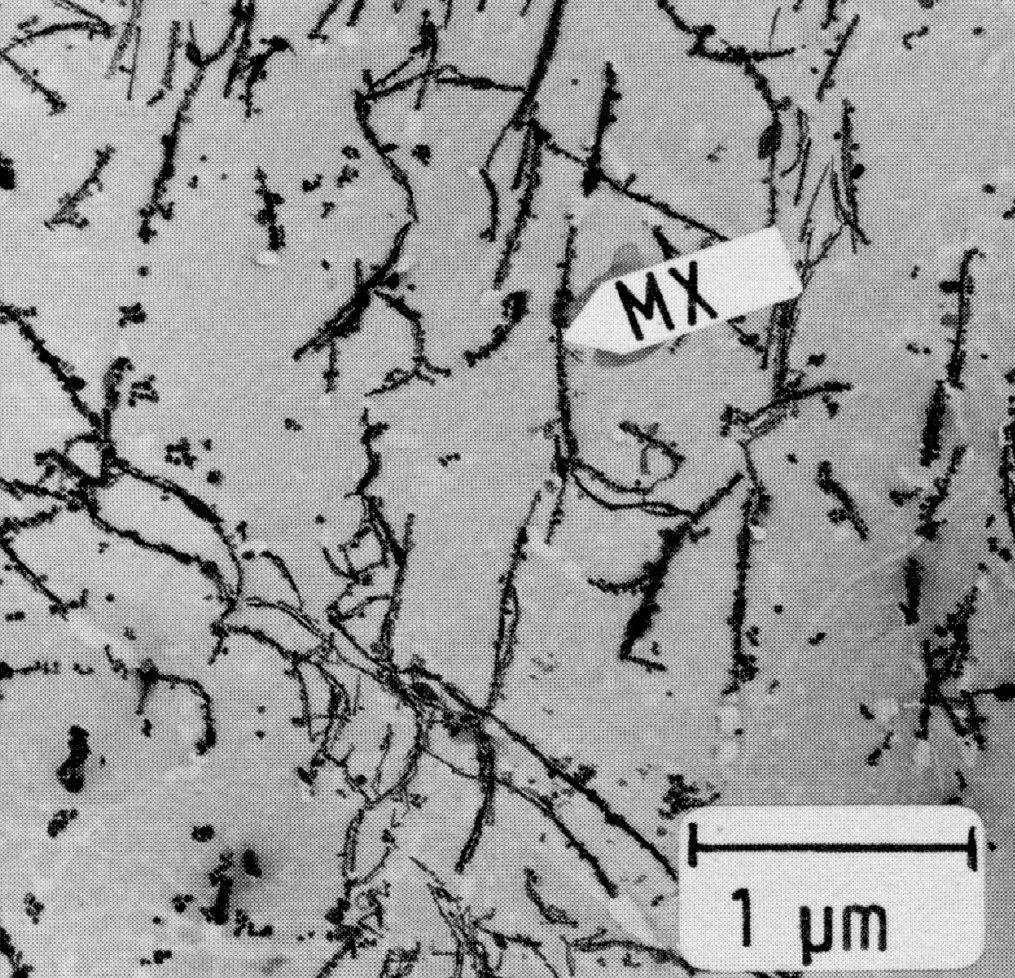
Figure 29: X10CrNiNb18-9, 1300 °C 15 min/W + 650 °C 100 h/air + 900 °C 5 h/air, extraction replica. Scale bar: 0.5 µm.
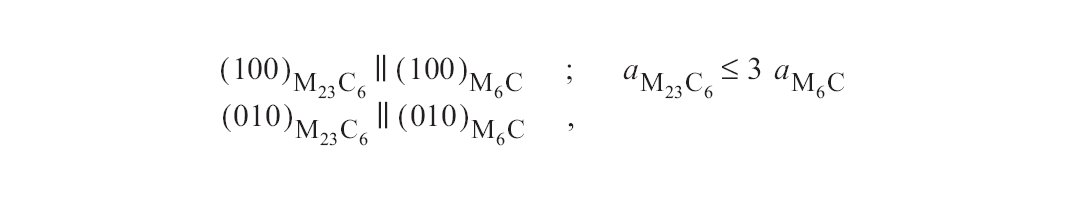
Figure b: There is evidence that the reaction M23C6 -> M6C takes place in situ, whereby the following orientation relationship holds between the two carbide phases.
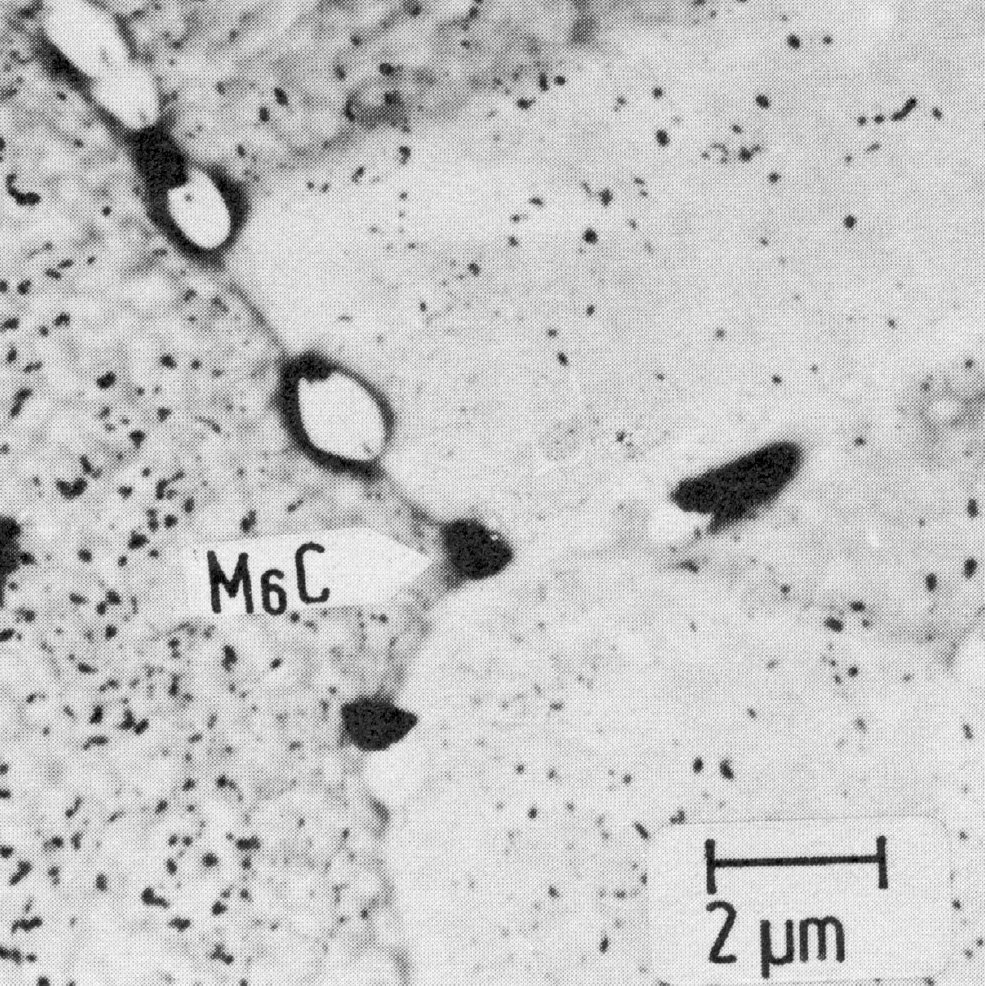
Figure 30: 16.6% Cr, 12.9% Ni, 2.0% Mo, 2.1% Nb, 1100 °C 1 h/W + 800 °C 1000 hair, extraction replica. Scale bar: 2 µm.
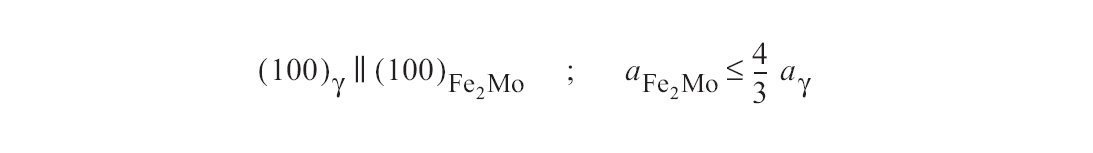
Figure c: Rod-shaped precipitates are typical for the Laves phase (Fe2Mo) (Fig. 33). The nucleation is partially coherent, with the orientation relationship.
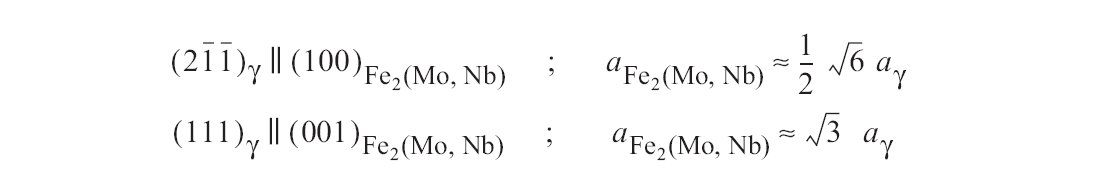
Figure d: At higher niobium contents, precipitates of a Laves phase, containing niobium, in the form of hexagonal plates is observed Fig. 34). These form by coherent nucleation with the orientation relationship.

Figure 31: 16.6% Cr, 12.9% Ni, 2.0% Mo, 2.1% Nb, 1100 °C 1 h/W + 800 °C 1000 h/air, extraction replica. Scale bar: 2 µm.
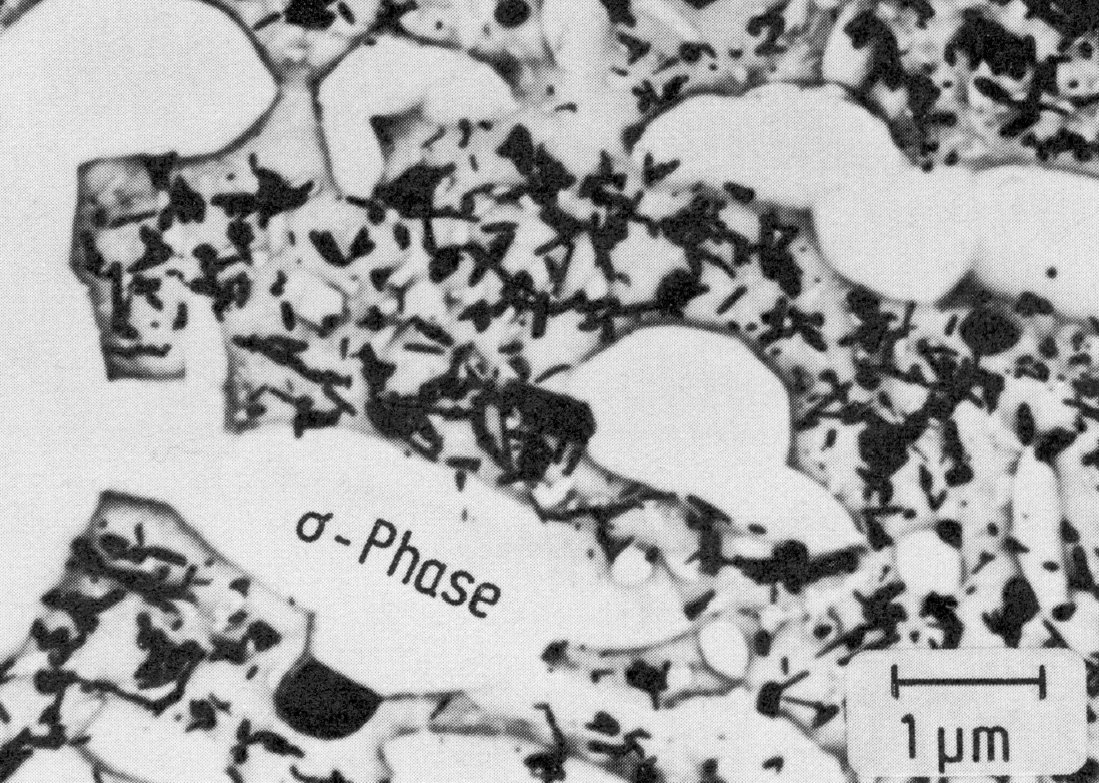
Figure 32: 17.4% Cr, 12.9% Ni, 4.6% Mo, 2.1% Nb, 1100 °C 1 h/W + 700 °C 10000 h/air, Sigma-phase (replica, not extracted). Scale bar: 1 µm.

Figure 33: 17.4% Cr, 12.9% Ni, 4.6% Mo, 2.1% Nb, 1100 °C 1 h/W + 600 °C 10000 h/air, extraction replica. Scale bar: 1 µm.

Figure 34: 16.7% Cr, 12.9% Ni, 2.1% Mo, 2.1% Nb, 1100 °C 1 h/W + 700 °C 1000 h/air, extraction replica. Scale bar: 1 µm.
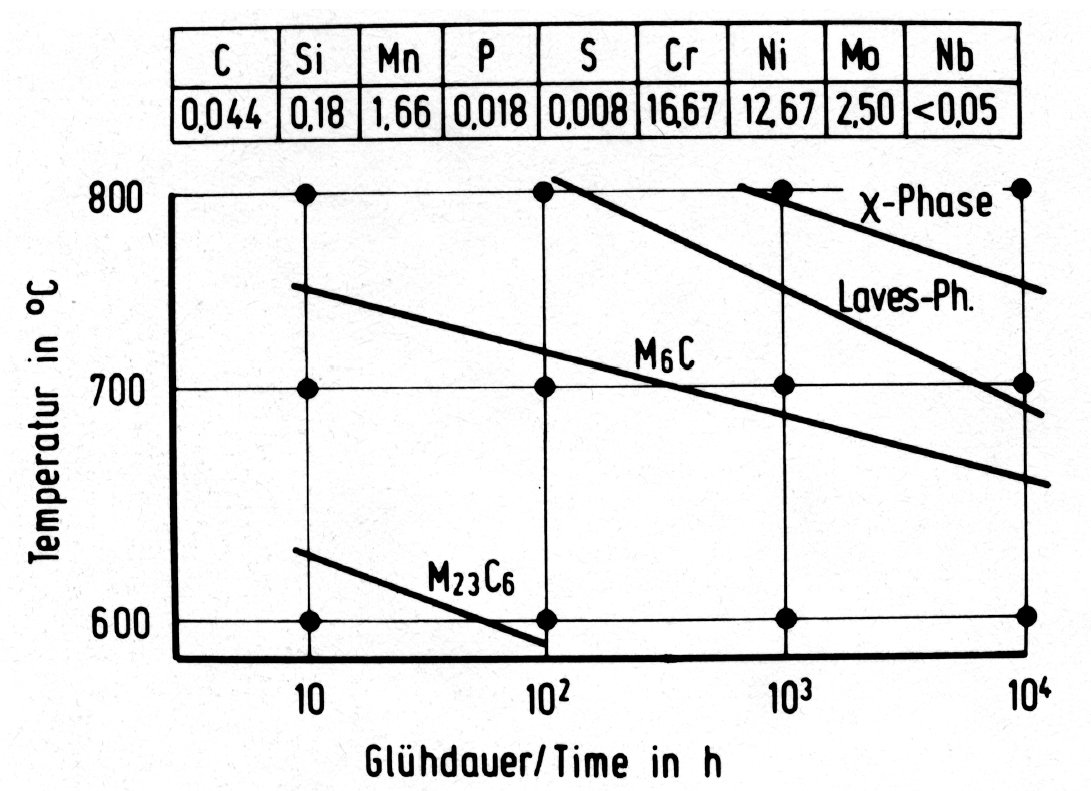
Figure 35: Time-temperature-precipitation diagrams for austenitic chromium-nickel steels with additions of molybdenum and niobium.
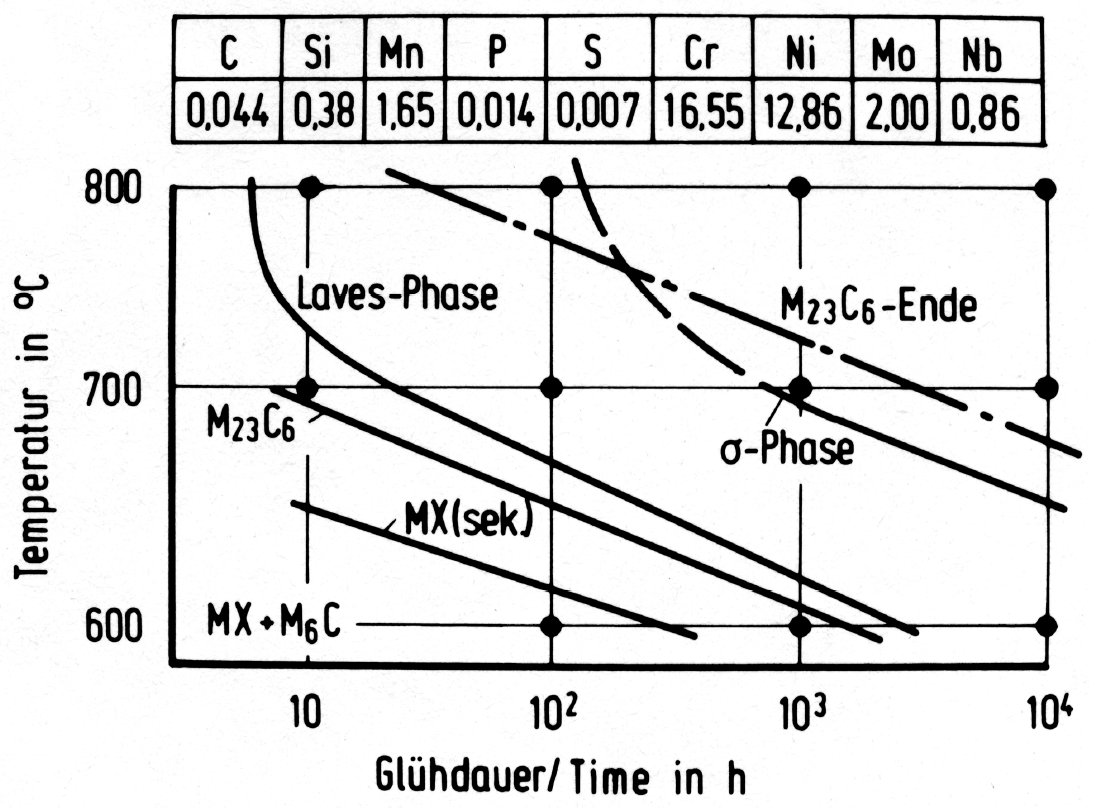
Figure 36: Time-temperature-precipitation diagrams for austenitic chromium-nickel steels with additions of molybdenum and niobium.
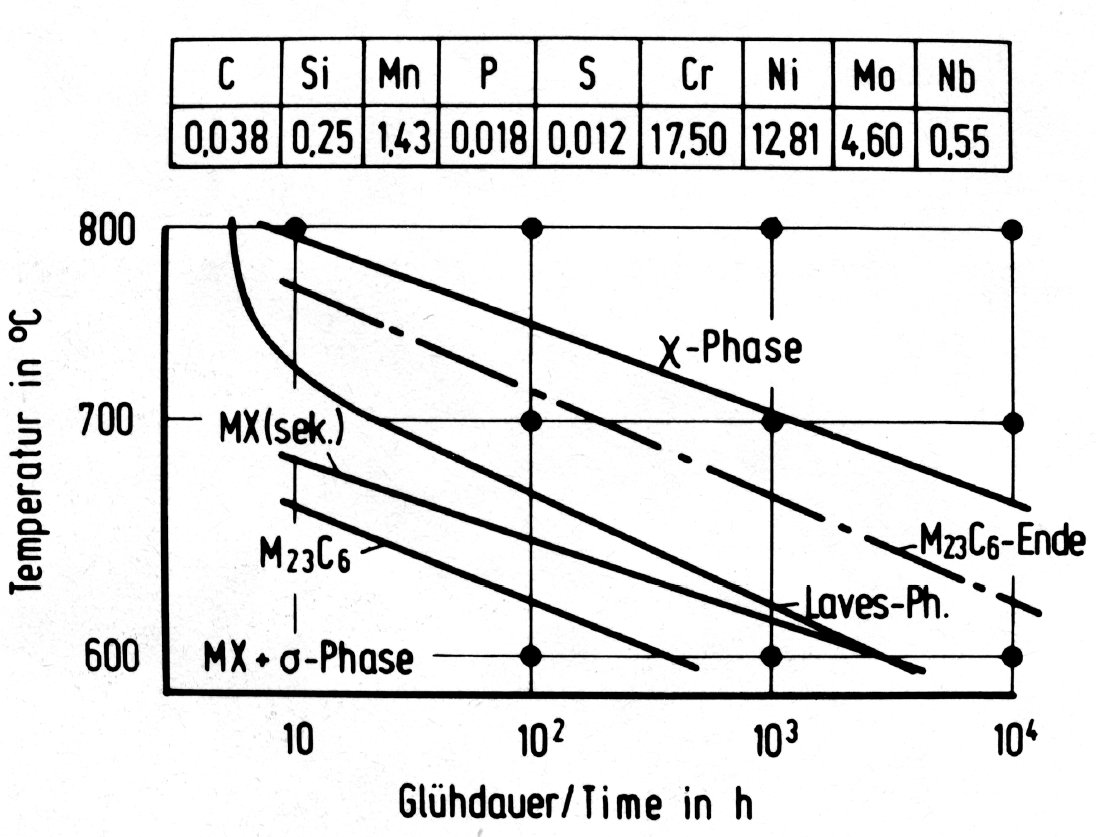
Figure 37: Time-temperature-precipitation diagrams for austenitic chromium-nickel steels with additions of molybdenum and niobium.
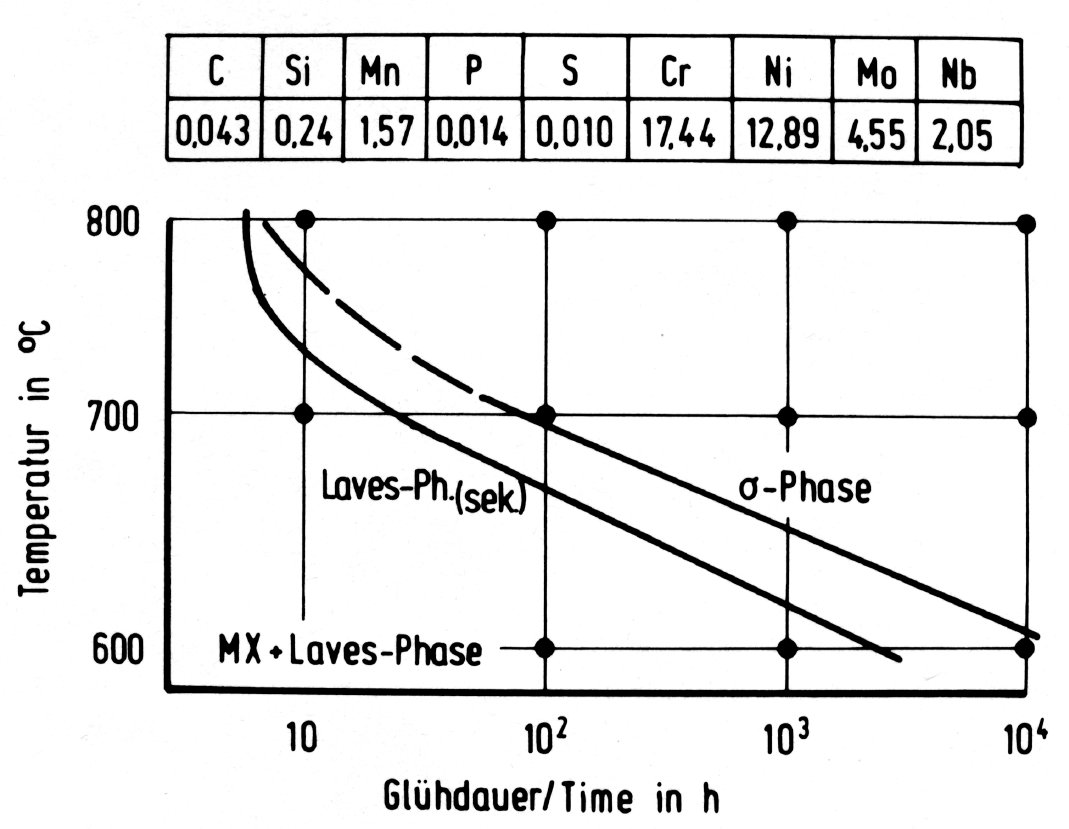
Figure 38: Time-temperature-precipitation diagrams for austenitic chromium-nickel steels with additions of molybdenum and niobium.

Figure 39: X10CrNiMoTi18-10, cooled without deformation, extraction replica. Scale bar: 0.5 µm.

Figure 40: X10CrNiMoTi18-10, hot- and cold-worked on cooling; Ti(C,N), M23C6, Fe2Mo, extraction replica. Scale bar: 0.25 µm.
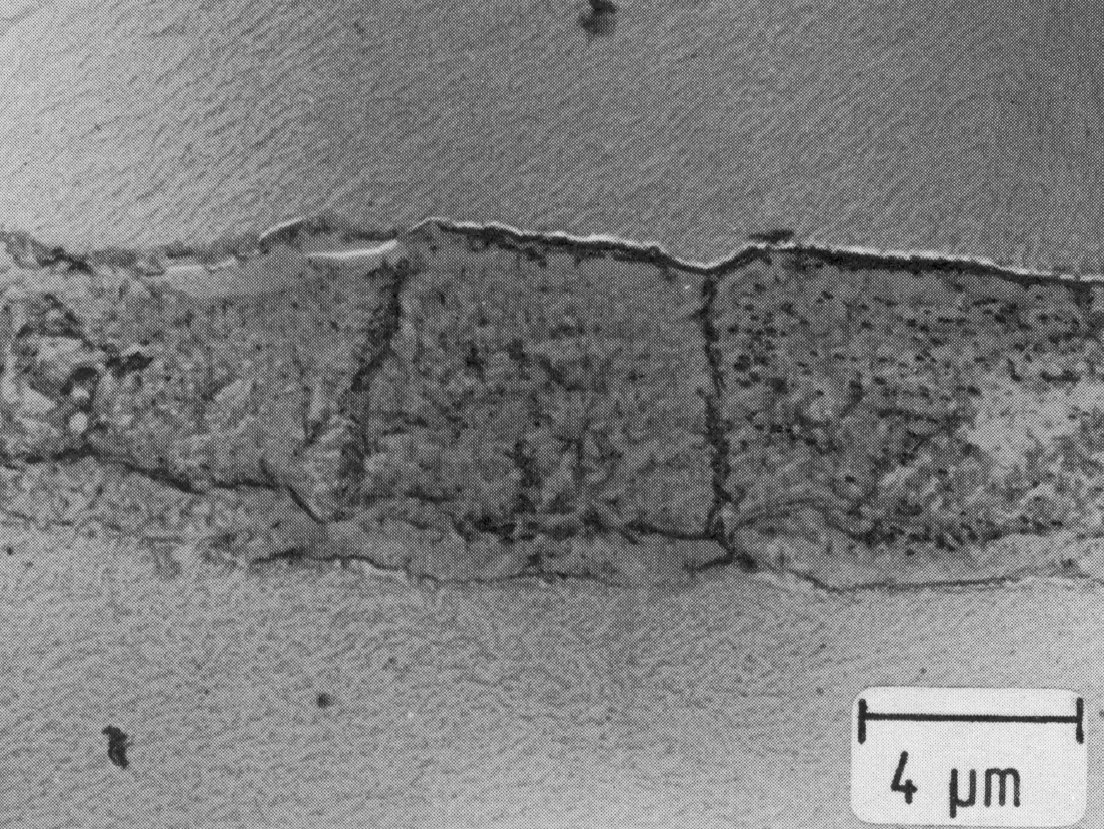
Figure 41: High-temperature ferrite in X10CrNiTi18-9 with MX precipitates, extraction replica. Scale bar: 4 µm.

Figure 42: Cr2N (tetragonal) electron diffraction pattern.
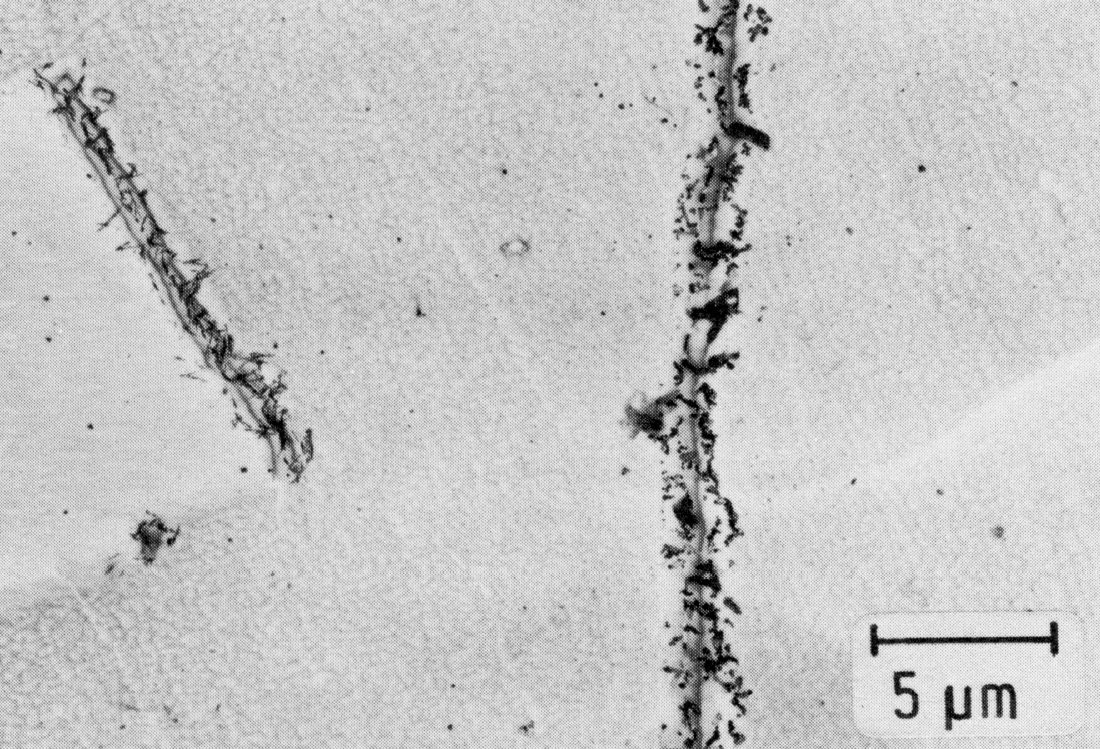
Figure 43: 0.02% C, 21% Cr, 40% Ni, 0.22% N, 1150 °C 30 min/W + 750 °C 6 min/WCr2N, little M23C6, extraction replica. Scale bar: 5 µm.
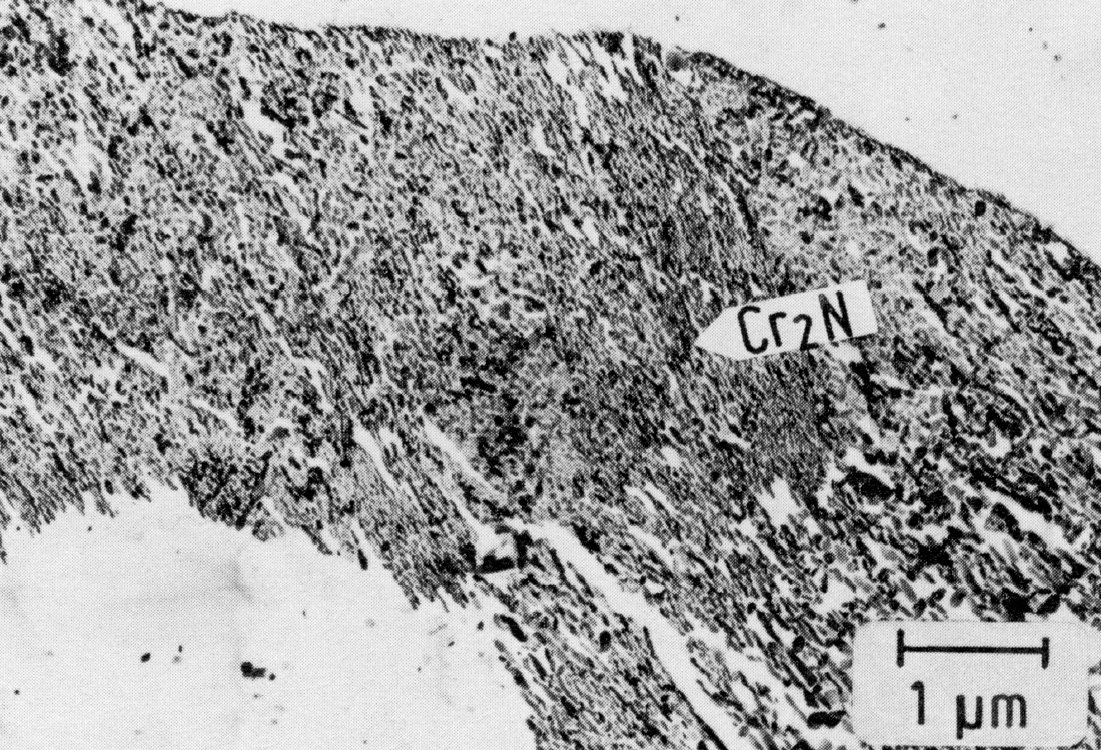
Figure 44: 0.02% C, 21% Cr, 40% Ni, 0.22% N, 1150 °C 30 min/W + 750 °C 30 min/W, extraction replica. Scale bar: 1 µm.
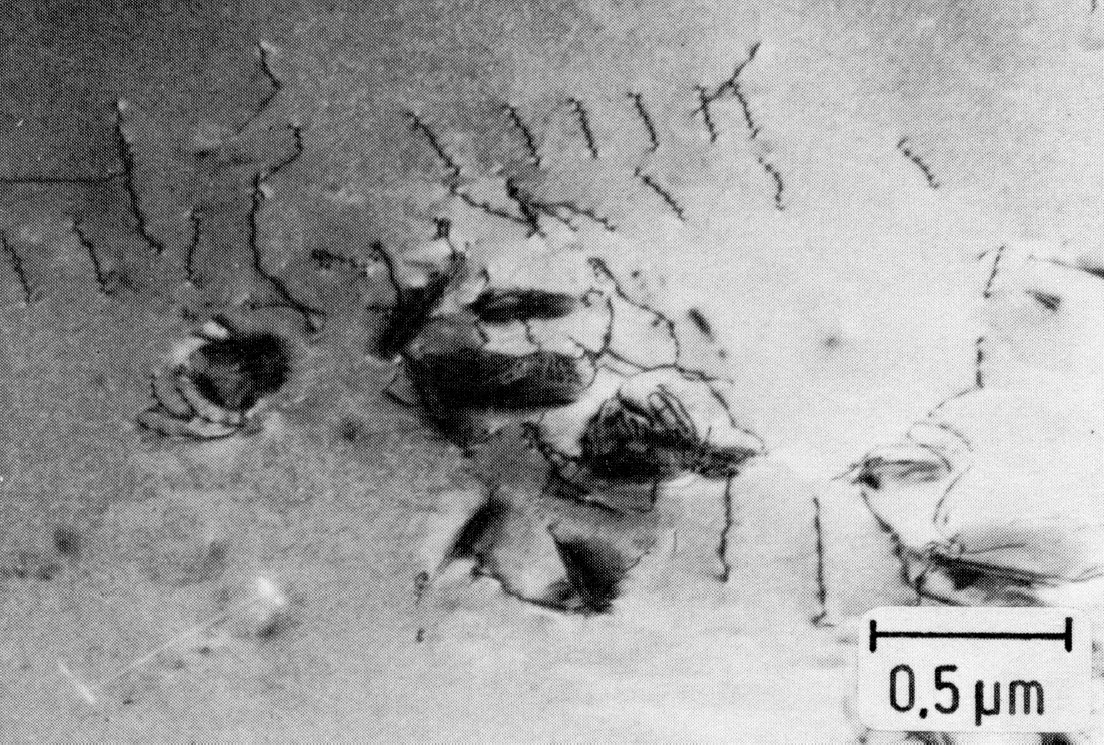
Figure 45: 0.02% C, 21% Cr, 40% Ni, 0.22% N, 1150 °C 30 min/W + 750 °C 5 h/W, thin foil. Scale bar: 0.5 µm.
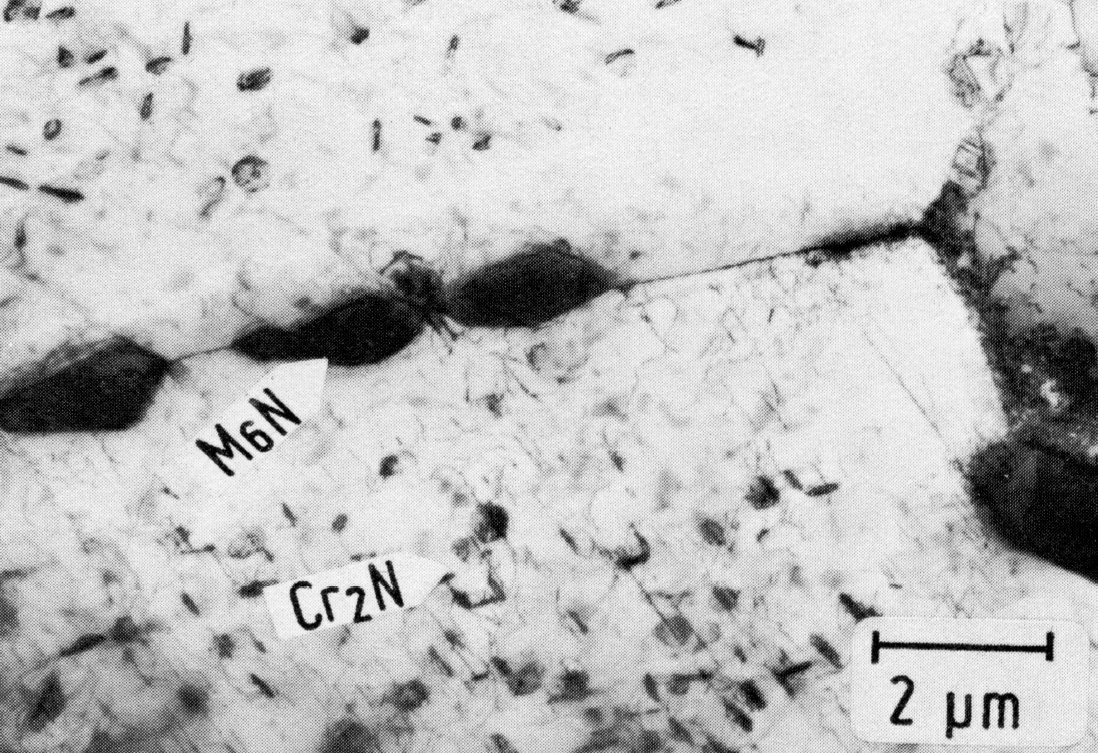
Figure 46: 0.02% C, 21% Cr, 40% Ni, 0.22% N, 1150 °C 30 min/W + 750 °C 2000 h/W, thin foil. Scale bar: 2 µm.
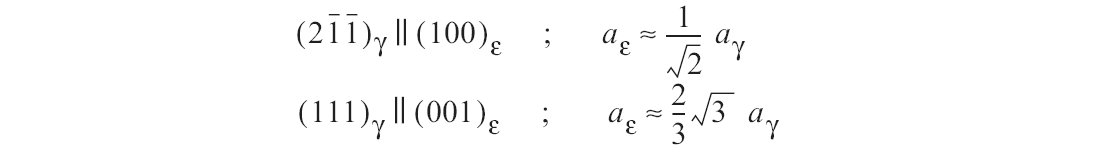
Figure e: Epsilon-martensite forms without diffusion by a shear parallel to the (111) planes. The orientation relationship between austenite and epsilon-martensite is.
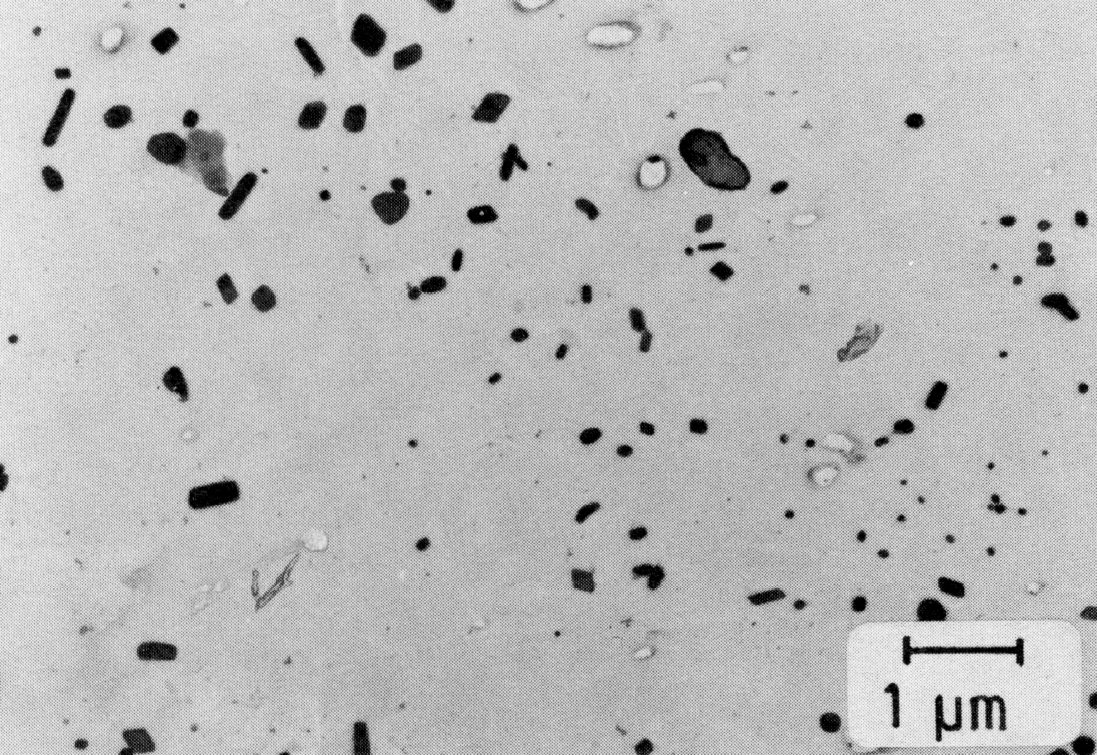
Figure 47: X3CrNiMoNbN23-17 + 0.046% Mg, 1150 °C 30 min/W; 2-phase, epsilon-phase, extraction replica. Scale bar: 1 µm.
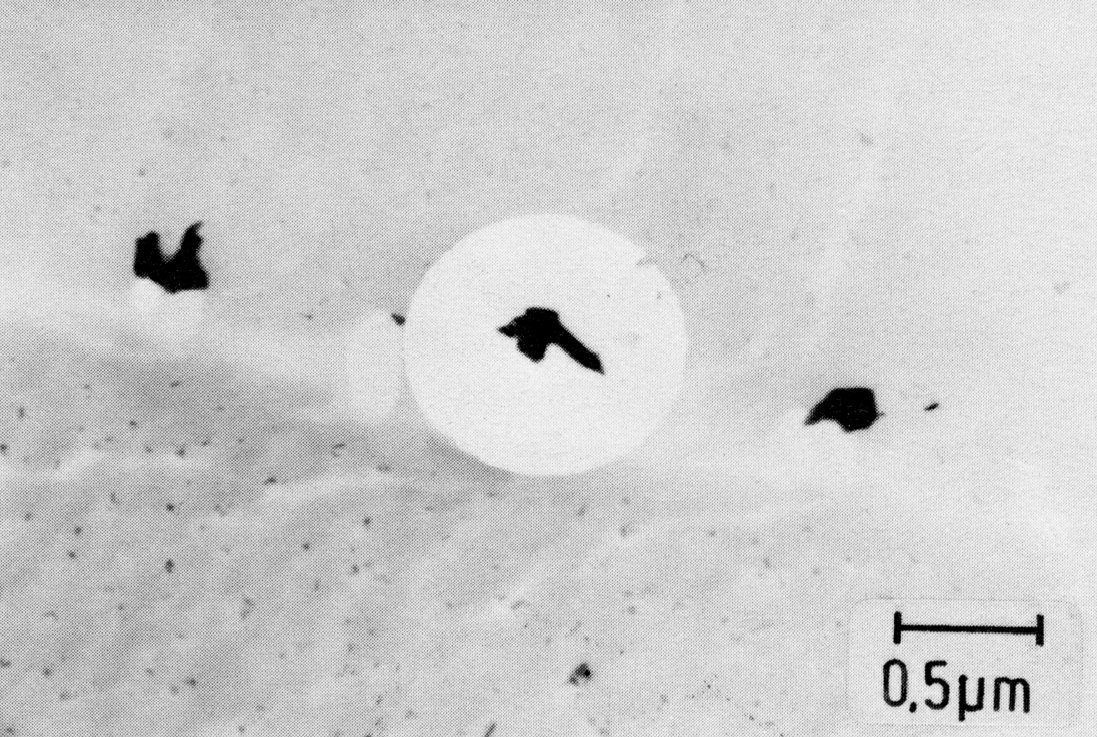
Figure 48: X6CrNi1613 + 0.009% B, 1200 °C 2 h/W; M2B, M23C6, extraction replica. Scale bar: 0.5 µm.
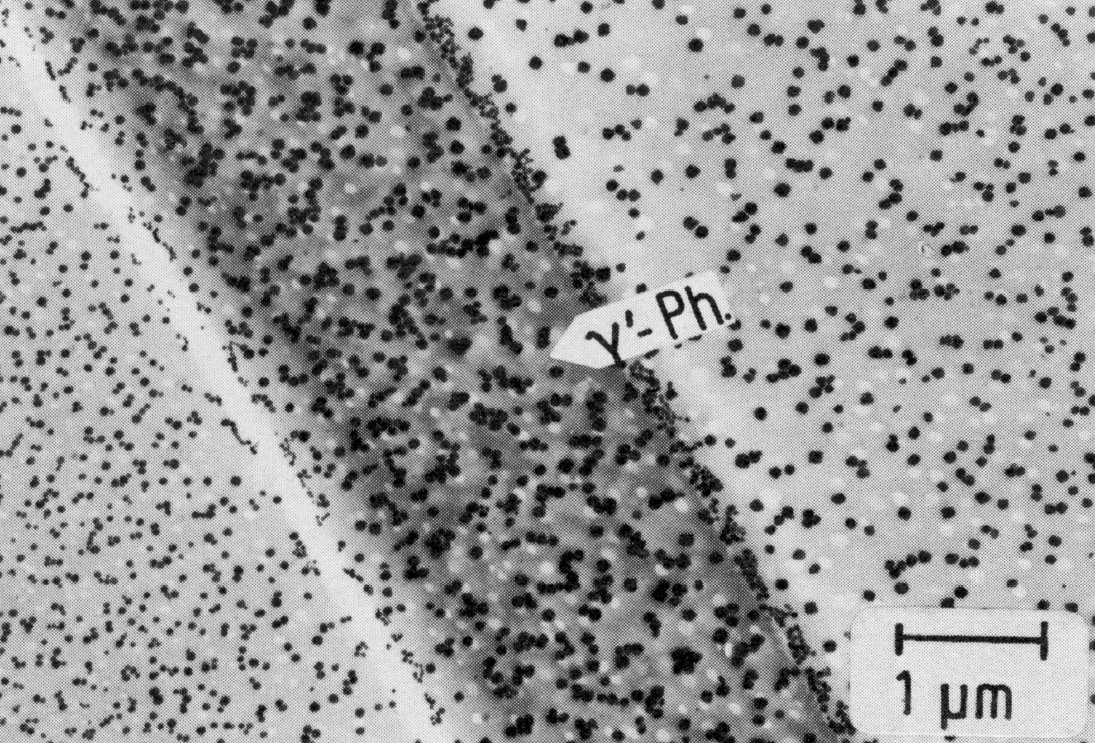
Figure 49: X5NiCrTi26-15, 980 °C 1 h/W + 760 °C 16 h/air, extraction replica. Scale bar: 1 µm.

Figure 50: X5NiCrTi26-15, 980 °C 1 h/W + 720 °C 16 h/air, extraction replica. Scale bar: 0.5 µm.
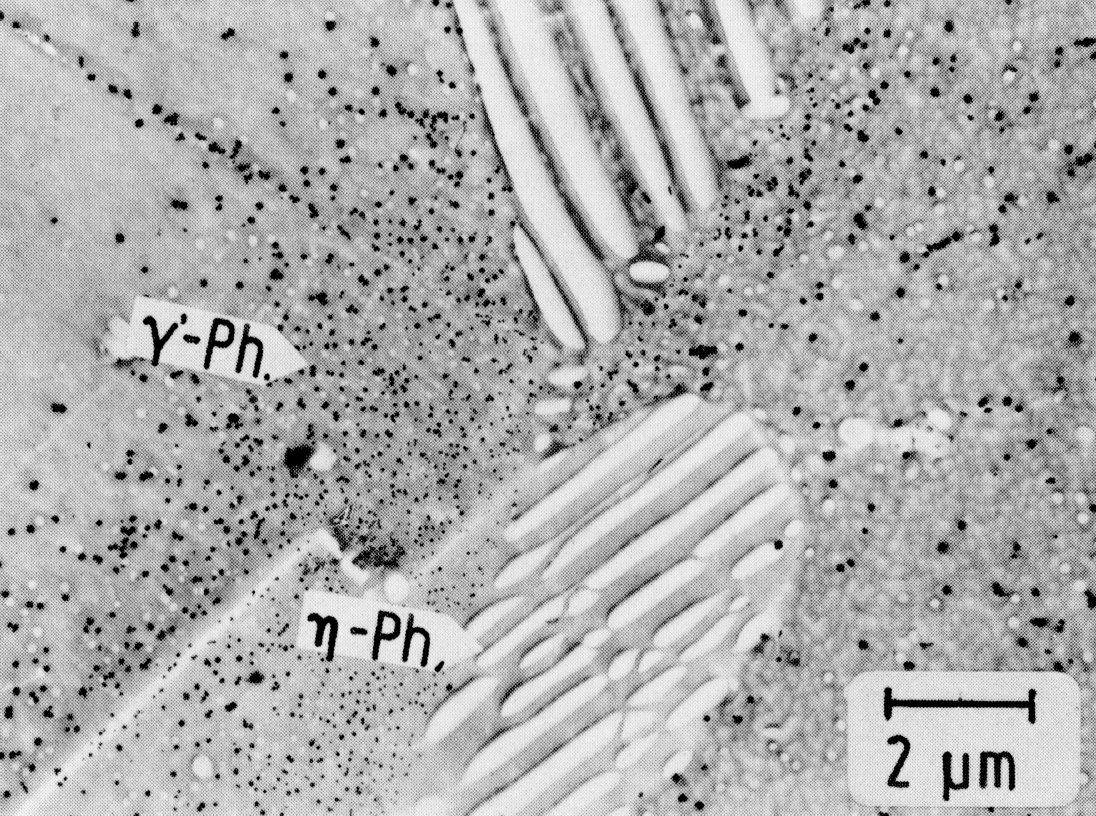
Figure 51: X5NiCrTi26-15, 980 °C 1 h/W + 760 °C 16 h/air, ç-phase, not extractable extraction replica. Scale bar: 2 µm.
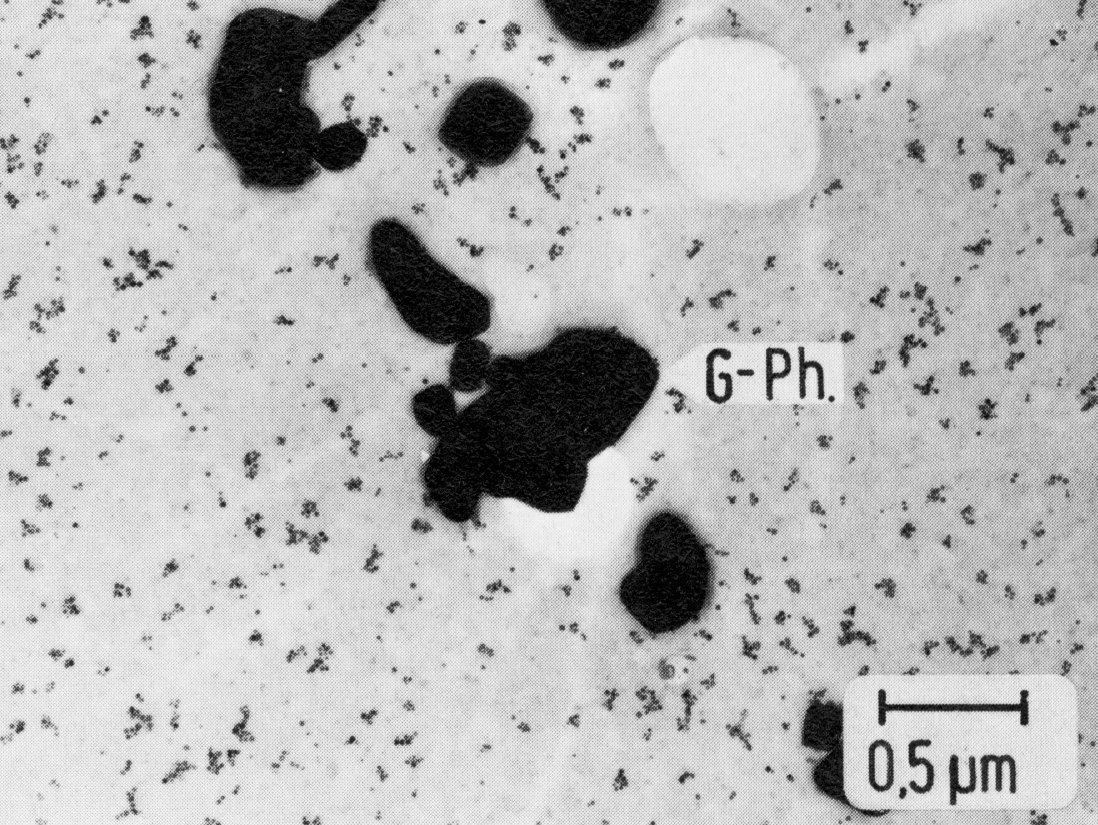
Figure 52: X5NiCrTi26-15, 980 °C 1 h/W + 720 °C 16 h/air, extraction replica. Scale bar: 0.5 µm.
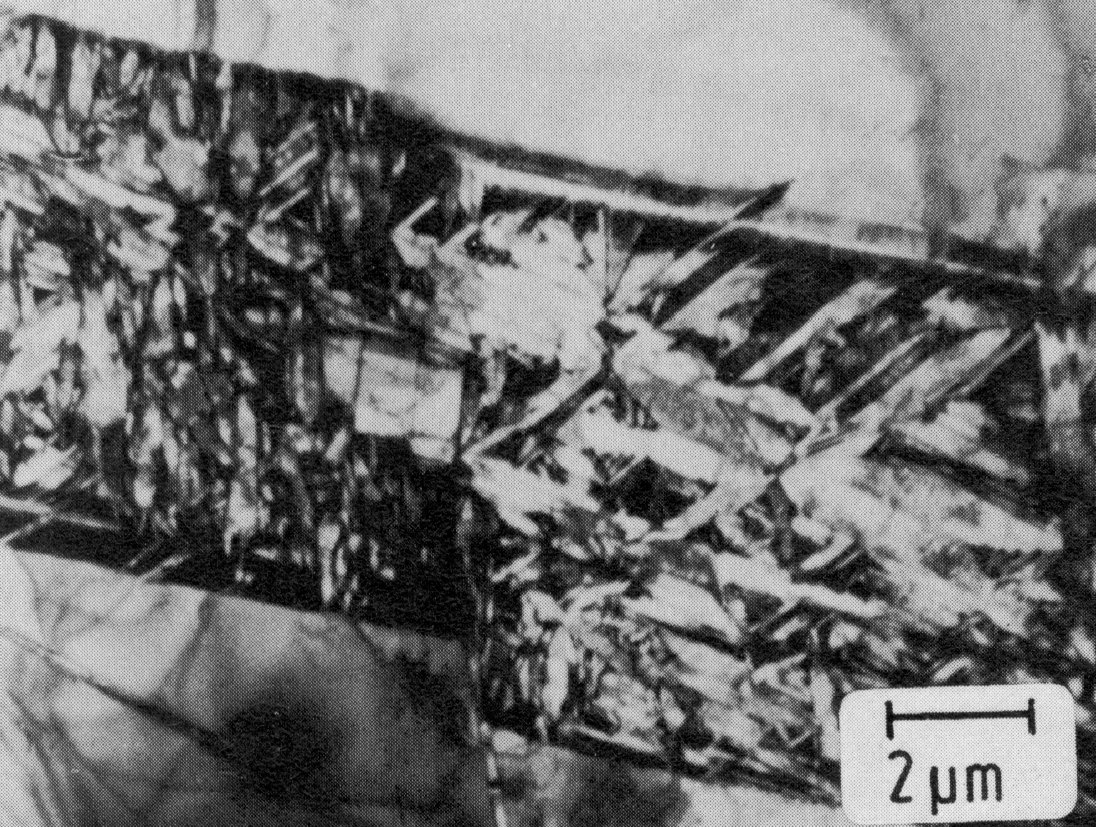
Figure 53: 17.3% Cr, 9.6% Ni, 0.8% Mo, 0.4% Ti, transformation of epsilon-martensite to alpha'-martensite, thin foil. Scale bar: 2 µm.

Figure 54: X12CrNi17-7, cold rolled, extraction replica. Scale bar: 5 µm.
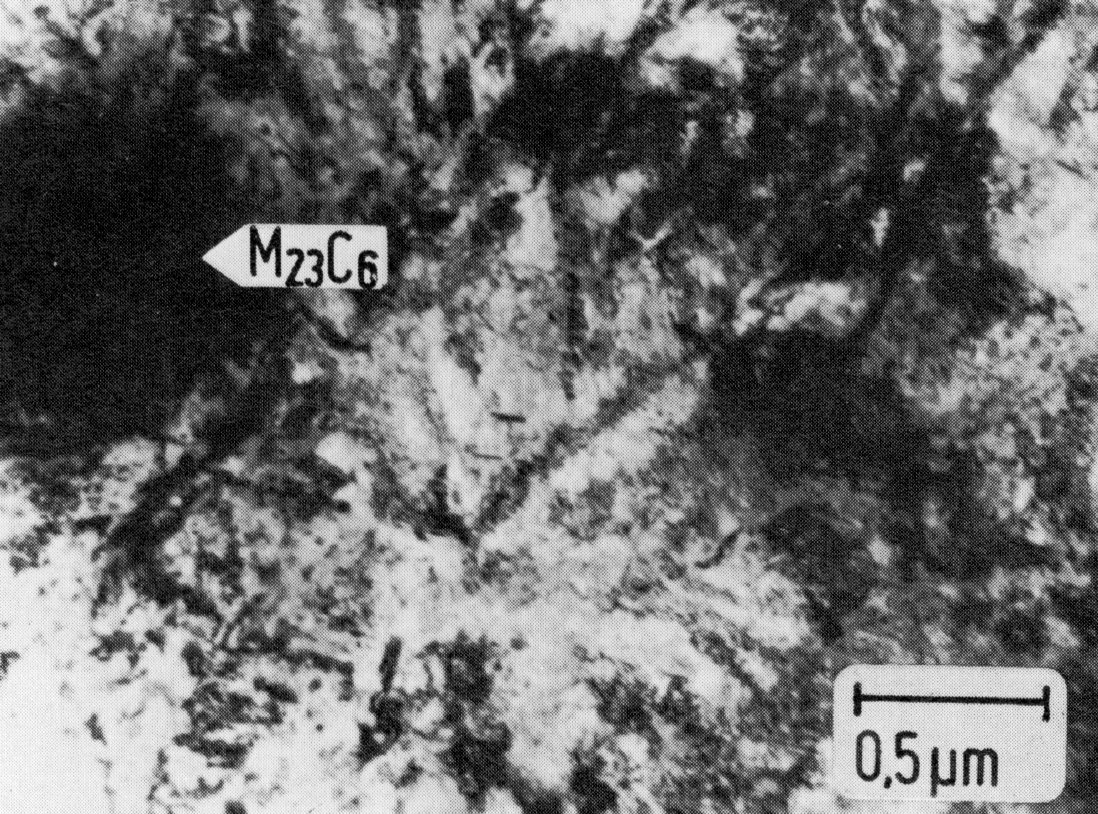
Figure 55: X12CrNi17-7, cold rolled + 420 °C 1 h/air, thin foil. Scale bar: 0.5 µm.
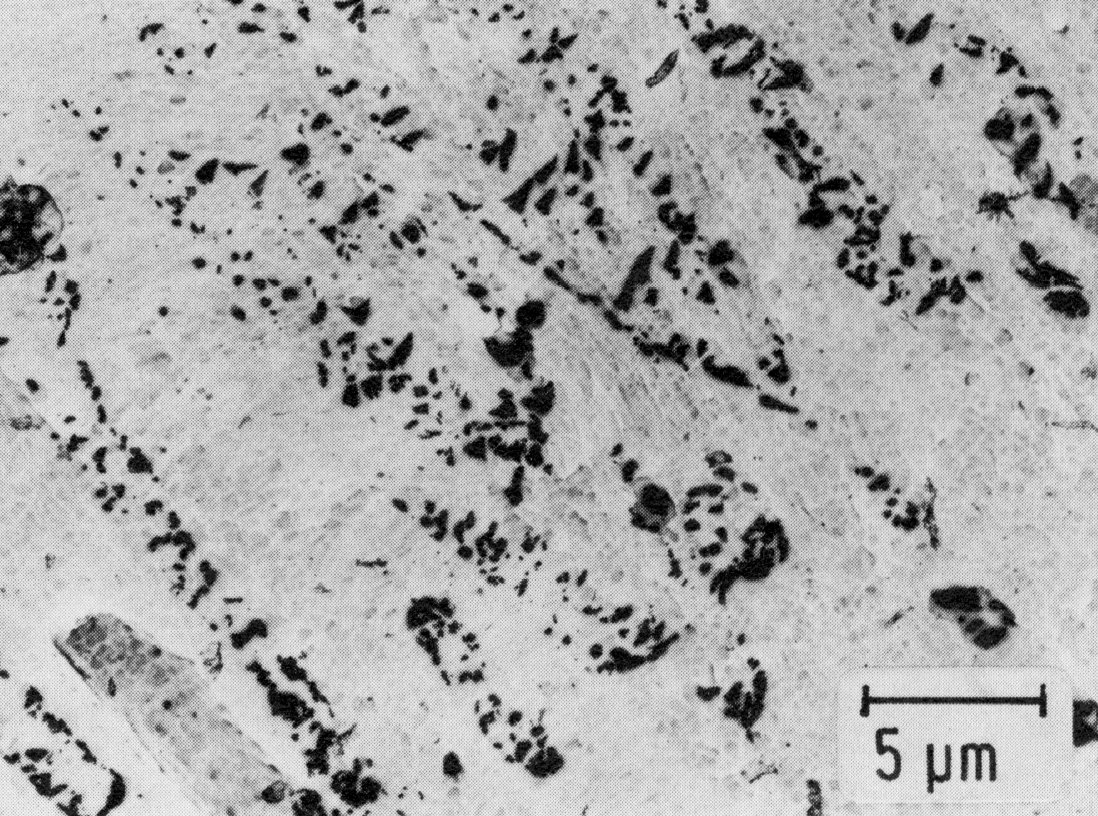
Figure 1: X12CrNi17-7, cold rolled + 420 °C 1 h/air, extraction replica. Scale bar: 5 µm.
Carbide name: See the text
Record No.: 1496
Carbide formula: See the text
Carbide type: See the text
Carbide composition in weight %: No data
Image type: TEM
Steel name: Austenitic steel
Mat.No. (Wr.Nr.) designation: No data
DIN designation: No data
AISI/SAE/ASTM designation: No data
Other designation: No data
Steel group: Austenitic steels
Steel composition in weight %: See the text
Heat treatment/condition: See the text
Note: In austenitic steels the austenite range is extended at least to room temperature, mostly to even lower temperatures, by suitable additions of chromium and nickel. The main groups are the stainless or acid-resistant steels with 18% Cr and 10% Ni, the high-temperature steels with 16% Cr and 13% Ni and also 16% Cr and 16% Ni and the heat resistant steels with up to 25% Cr and 35% Ni. In addition, there are numerous steels with other chromium and nickel contents, of which two small, but important groups may be mentioned: high-temperature steels hardenable by intermetallic phases and metastable steels with 18% Cr and 8% Ni, which form martensite on cooling to low temperatures and/or on cold working. The spring steels belong to the last group.
Apart from the two main alloying elements, all austenitic chromium-nickel steels contain up to 2% Mn. Higher manganese contents and decreasing nickel contents encourage the formation of martensite at low temperatures. Other additions, which either directly contribute to the formation of precipitates or extend the range of precipitation and accelerate the reaction, are up to about 1% Nb, 2% Ti, 5% Mo, 0.2% N, 0.015% B, 2% Si and 1.5% Al. The carbon content is generally 0.1% or less.
Despite the considerable differences in the chemical compositions of these steels, the precipitation processes occurring in austenitic chromium-nickel steels show great similarities. The most important precipitate phase is the face-centred cubic chromium carbide M23C6. It occurs in almost all austenitic steels as a stable, or at least metastable precipitate.
Fig. 22 shows the time-temperature-precipitation diagram of the steel X5CrNi18-9 as an example. The precipitation always starts on the grain boundaries, the form of the M23C6 precipitates depending to a large extent on the heat treatment. On slow cooling from high temperatures, M23C6 precipitates as a rule in the form of dendritic particles (Fig. 23). Heat treating at 800 °C or above often leads to the formation of single particles with simple geometrical forms on and near the grain boundaries Fig. 24). Precipitation treatments at temperatures below 800 °C lead to the formation of mostly triangular, plate-shaped particles on the grain boundaries. After relatively short times the grain boundaries show a high density of these particles (Fig. 25). This precipitation begins on the normal grain boundaries. Later the incoherent twin boundaries and finally the coherent twin boundaries are also occupied. Grain boundary precipitates of M23C6 formed below 800 °C lead to a marked chromium depletion in the region of the grain boundary and hence an increased sensitivity to intergranular corrosion.
M23C6 often precipitates with coherent nucleation in the austenite. The orientation relationship is written in Fig. a.
To avoid liability to intergranular corrosion, chromium-nickel steels are produced with carbon contents below 0.03% or stabilized with niobium or titanium. With sufficient additions of the stabilizing elements and correct heat treatment (1050 °C/water), practically all the carbon is bound in the form of relatively coarse, globular carbonitrides of the type MX. The carbonitrides should be distributed statistically throughout the microstructure. However, MX is partially dissolved by heat treating at high temperatures, and subsequent aging leads to precipitation of new MX and (metastable) M23C6.
Fig. 27 shows the time-temperature-precipitation diagram of the steel X10CrNiNb-18-9 after solution treatment at 1300 °C/water as an example. It is seen that only MX is precipitated by aging above 900 °C. The particles are granular or in the form of threads (Fig. 28 and Fig. 29), the latter increasing at the expense of the former as the temperature is lowered. The MX threads form along dislocation lines. At all low temperatures MX particles are found within the grains and M23C6 precipitates on the grain boundaries. At temperatures below 750 °C M23C6 starts to precipitate before MX.
The precipitation sequence in titanium-stabilized steels is quite similar, so that a detailed description may be omitted. A typical feature of these steels is the presence of cubic titanium nitrides of the type MX, which precipitate from the melt and may easily be recognized by their characteristic yellow colour in the light microscope. However, these titanium nitrides are also introduced into the titanium alloyed, austenitic chromium-nickel steels through the addition of scrap to the melt. In titanium-stabilized steels, the titanium nitrides serve as nuclei for the precipitation of carbon-rich titanium carbonitrides, visible as a grey border. The titanium carbides are re-dissolved before the solidus temperature is reached.
With regard to the precipitation behaviour, molybdenum is an interesting alloy element in austenitic steels, for additions of this element cause the dissolution of the M23C6 first precipitated in favour of the ternary face-centred cubic carbide M6C. The latter is formed mainly as relatively coarse, globular particles on the grain boundaries (Fig. 30). There is evidence that the reaction M23C6 ¨ M6C takes place in situ, whereby the following orientation relationship holds between the two carbide phases (Fig. b).
Molybdenum also encourages the formation of the tetragonal Sigma-phase. This phase, with the basic composition FeCr can also arise in molybdenum-free steels at temperatures below 800 °C. In niobium-stabilized steels the range of stability of the Sigma-phase is hardly changed, whereas it is extended and displaced to higher temperatures by additions of titanium and/or molybdenum. Silicon, which does not form its own Sigma-phase, enlarges the range and amount of the precipitation to such a degree that in a steel with 25% Cr, 20% Ni and 2% Si the Sigma-phase can also occur in the form of long needles with preferred orientation in the austenite.
In steels containing molybdenum, the body-centred cubic ÷-phase and the hexagonal Laves phase may also occur. The ÷-phase, e.g. Cr12Fe36Mo10 also forms on the grain boundaries (Fig. 31). The precipitates are, however, generally finer than those of the ó-phase (Fig. 32). Rod-shaped precipitates are typical for the Laves phase (Fe2Mo) (Fig. 33). The nucleation is partially coherent, with the orientation relationship (Fig. c).
At higher niobium contents, precipitates of a Laves phase, containing niobium, in the form of hexagonal plates is observed Fig. 34. These form by coherent nucleation with the orientation relationship (Fig. d). The habit plane is the (001) plane of the Laves phase.
The three intermetallic phases discussed above can occur together in molybdenum-alloyed austenitic steels. The order of formation, the range of stability and the relative amounts are, however, very sensitive to the chemical composition and possibly also to the solution treatment preceeding the precipitation heat treatment. This is shown for example in the time-temperature-precipitation diagrams in Fig. 35 to Fig. 38.
These precipitation processes can be considerably accelerated by a so-called hot-cold deformation, as shown as an example in the case of the steel X10CrNiMoTi18-10. After a cooling time of 17 min between 1265 and 600 °C, only grain boundary precipitates of Ti(C,N) are found (Fig. 39). The same cooling with rolling at temperatures down to below 900 °C leads to fine precipitates in the grains, which may be attributed to the phases Ti(C,N), M23C6 and Fe2Mo (Fig. 40).
Depending upon the alloy composition, many of these steels can contain high-temperature ferrite as a metastable, and sometimes as a stable phase (Fig. 41). M23C6 forms on the austenite-ferrite boundaries and starting from these points the ferrite decomposes into austenite and ó-phase. Since the formation of the ó-phase takes place considerably faster in ferrite than in austenite, a chromium depletion of the newly-formed austenite may occur.
To increase the solid solution strength of the austenite, it is possible to add up to 0.2% N to the open-melted chromium-nickel steels. To avoid intergranular corrosion the carbon content should be below 0.03 %. Accordingly, very little M23C6 and a great amount of hexagonal Cr2N is formed on aging. The latter phase belongs, like Fe2N and Mo2C, to the M2X group. In addition to the hexagonal form, a tetragonal Cr2N has also been found (Fig. 42).
The precipitation of Cr2N takes place, as in the case of M23C6, first on grain boundaries and then on twin boundaries (Fig. 43). Cr2N can also occur as a discontinuous precipitation Fig. 44). Within the grains, the nucleation takes place on dislocations. Since dislocations arise in the surrounding austenite during the precipitation of plate-shaped Cr2N particles, (Fig. 45), new precipitates form preferentially near these platelets. For this reason, the precipitation process, starting from the grain boundary, proceeds only very slowly into the interior of the grains and causes no hardening.
In steels containing nitrogen with 18% Cr and < 30% Ni, 20% Cr and 20% Ni or 25% Cr and < 25% Ni, Cr2N is the stable precipitate18). At higher nickel contents the Cr2N re-dissolves, at least partially. In its place, Cr3Ni2SiN, a cubic M6N , corresponding to M6C with a lattice parameter of 1.06 nm, forms preferentially on the grain boundaries. This rapidly coarsening nitride containing silicon even occurs in steels with only 0.3 to 0.5 % Si. It may be assumed that M6N is the stable phase, and not Cr2N (Fig. 46).
Small amounts of boron are added to creep-resistant steels to improve the grain-boundary strength. In steels with about 16% Cr and 13% Ni, not more than 0.015% B should be added on account of the lowering of the solidus line20). After quenching from high temperatures, steels containing boron show grain boundary precipitates (Fig. 48) consisting of M23C6 with boron and orthorhombic (Fe,Cr)2B. These two phases are so similar in appearance that they can be distinguished only by electron diffraction.
Austenitic chromium-nickel steels with additions of aluminium and/or titanium can harden by precipitation of the cubic gamma'-phase Ni3(Al,Ti)21) (Fig. 49, cf. also 5). This phase is stable only at sufficiently high aluminium contents. Otherwise it re-dissolves in favour of the hexagonal ni-phase Ni3Ti (Fig. 50), which often precipitates discontinuously on grain and twin boundaries (Fig. 51). In addition, the face-centred cubic G-phase (e.g. Ni16Si7Ti6, see also23) ) may form (Fig. 52), which is very similar to the phase M6(C,N) described above with regard to the crystal structure and partly also on account of the silicon content. For the sake of completeness it may be mentioned that a further phase containing silicon with the same crystal structure the so-called S-phase has been reported in the literature.
In many austenitic chromium-nickel steels the austenite is not stable. By cold working or cooling to low temperatures various amounts of tetragonally distorted alpha'-martensite may be produced. In addition, the hexagonal epsilon-martensite may also occur. Epsilon-martensite forms without diffusion by a shear parallel to the (111) planes. The orientation relationship between austenite and epsilon-martensite is (Fig. e).
Corresponding to the mechanism of formation, epsilon-martensite occurs in the form of plates with the habit plane (111)gamma || (001)epsilon. The epsilon- martensite generally transforms by a shear mechanism - except in steels with high manganese contents - to alpha'-martensite, so that numerous fine alpha'-martensite needles are found within the original epsilon-martensite plates (Fig. 53).
The spring steels, e.g. X12CrNi17-7 (Material No. 1.4310) are among those with unstable austenite. This steel is austenitic after rapid cooling from high temperatures. It becomes mainly martensitic only when cold worked. To attain the desired tensile strength, it may be necessary to interrupt the cold rolling and heat the steel for a short time to 400 °C. The precipitation of M23C6 can occur on the martensite needle boundaries already during this intermediate heat treatment (Fig. 54). However, these precipitates can be detected with certainty only in extraction replicas, since in transmission the high dislocation density causes so much diffraction contrast even around the carbides that the fine carbides cannot be seen, and the coarse carbides only with difficulty (Fig. 55). The carbide precipitation increases on aging after cold rolling (Fig. 56). Residual austenite with a high dislocation density is also present.
Links: No data
Reference: Atlas of Precipitates in Steels, Verlag Stahleisen GmbH, Germany, December 1983, pp. 433-475.