
Alphabetical Index
Browse by Elements
Keyword Search
Dry Etchants
Dry and Wet Etchants
Wet Etchants
Bulk Etchants
Layer Etchants
Nano Etchants
Single Crystal Etchants
Thin Film Etchants
Thin Foil Etchants
Wafer Etchants
Al Etchants
Cd Etchants
Ga Etchants
Ge Etchants
In Etchants
New Etchants
Other Etchants
Si Etchants
Zn Etchants
Help
Home
ZrO2 Thin Film - Dry Etching
Material Name: ZrO2
Recipe No.: 10310
Primary Chemical Element in Material: Zr
Sample Type: Thin film
Uses: Etching
Etchant Name: None
Etching Method: Dry etching
Etchant (Electrolyte) Composition: The ZrO2 thin films used in this work were deposited by
atomic layer chemical vapor deposition. The total thicknesses of
the ZrO2 thin films were about 100 nm. The dry etching process
was performed in an ICP system, as shown schematically in Fig.
1. This consisted of a cylindrical chamber with a diameter of 26
cm. The top copper coil was operated with 13.56 MHz RF power,
and was located on the chamber lid, to generate high-density
plasma. The bottom electrode was connected to another 13.56
MHz asymmetric RF generator, to control the DC-bias voltage.
The distance between a quartz window and the substrate electrode
was 9 cm. The chamber was evacuated to 10 exp(-6) Torr using a
mechanical pump (2M80, BOC Edwards) and a turbo molecular
pump. The ZrO2 thin films were etched with CF4/Ar gas mixing.
The gas mixing ratio and process pressure were varied, to find the characteristics of etching. For these experiments, RF power,
DC-bias voltage, process pressure and substrate temperature
were 600 W, - 250 V, 15 mTorr and 45C, respectively. In addition,
plasma etching of ZrO2 thin films was investigated by including
the RF power, DC-bias voltage, and process pressure of 500 W ~
650 W, - 150 V ~ - 300 V, and 9 ~ 20 mTorr in the CF4/Ar gas mixing
ratio. The etch rates were measured using a depth profiler KLA
Tencor, alpha-step 500; Sanjoe, Ca, USA). The chemical reactions on
the surfaces of the etched ZrO2 thin films were evaluated using
X - ray Photoelectron Spectroscopy (XPS, Thermo VG, SIGMA
PROBE; East Grinstead, West Sussex, England). The source type
for the XPS analysis was Al K alpha with a spot size of 400 µm. The energy step size was 0.1 eV.
For the characterization of ZrO2 thin film in an ICP etching
system, the plasma etching of ZrO2 thin film and selectivity of
ZrO2 to SiO2 were systematically investigated as various etch
chemistries. Figure 2 shows the etch rate of ZrO2 thin film and
selectivity of ZrO2 to SiO2 as a function of reactive species concentration,
when the total flow rate was maintained at 20 sccm.
Other process conditions, such as RF power, DC-bias voltage,
process pressure, and substrate temperature, were maintained at
600 W, - 250 V, 15 mTorr, and 45C, respectively. Comparisons of
etch rates of the ZrO2 thin film in Ar- (25 nm/min) and CF4- (44.03 nm/min) based plasmas showed that the chemical etching was
more effective than physical sputtering [10]. The etch rate of
ZrO2 thin film increased, while the selectivity of ZrO2 to SiO2 also
increased. The maximum etch rate of ZrO2 was 60.8 nm/min at a
20% of CF4/(CF4+Ar) gas mixing ratio. It is well known that fluorine
components of ZrO2 thin film form high-volatile by-product,
such as ZrF4 (melting point: 450C). The evident enhancement
of the ZrO2 thin film etch rate in the pure CF4 plasmas allows
one to assume that the chemical etch pathway provided by the
F atom is the dominant mechanism for the given set of input
process parameters. In the case of the chemical etching of
the ZrO2 thin films, we expect the contribution of this pathway
to be much lower, compared with the ion assisted chemical reaction.
The reason for this is that for ion energies of about 300 - 500
eV, typical sputtering yields for metal oxides do not exceed 0.5 -
1 atom/ion. In CF4-based plasma, addition of the CF4 up to 20%
increased the etch rate through the action of two mechanisms: 1)
the accelerated chemical reaction by the ion-stimulated desorption
of the reaction products, and 2) increase of the contribution
of the chemical etching. Nevertheless, when the CF4 content exceeds
20%, the etch rate begins to fall, due to the gdisappearanceh
of the chemical etching.
Figure 3 shows the etch rate of ZrO2 thin film as a function of
RF power. Other process conditions, specifically CF4/Ar(20:80%)
plasma, DC-bias voltage, and process pressure, were also maintained
at - 250 V, and 15 mTorr. As RF power increases, the ZrO2
thin film also increases, starting from 35.7 nm/min at 500 W, and
then reaches a maximum of 68.08 nm/min at 650 W. It can be
seen that an increase in RF power causes a monotonic increase
in both dissociation and ionization rates, and thus, in the densities
and fluxes of F atoms and positive ions. In our case,
such layer can result from the deposition of solid F that is then
bonded with surface oxygen to form Zr-F, as well as from F radicals
incorporated in the polymer-like structure.
The etch rates of ZrO2 thin film are shown in Fig. 4 as functions
of the DC-bias voltage. Other process conditions, namely
CF4/Ar(20:80%) plasma, RF power and process pressure, were
also maintained, at 600 W, and 15 mTorr. As the DC-bias voltage
increases from - 150 to - 300 V, the etch rate of ZrO2 thin film
increases from 33.98 to 97.5 nm/min. The selectivity of ZrO2 to
SiO2 was slightly increased. An increase in etch rate can be related
to the increase of mean ion energy, resulting in increasing
sputtering yields for the ZrO2 thin film, and reaction products.
The effect of process pressure on etch rate is shown in Fig. 5. As process pressures increased from 9 to 20 mTorr, the etch
rates of ZrO2 decreased from 79.98 to 53.8 nm/min. However,
we obtain a similar non-monotonic behavior, as was mentioned
for the effect of the gas mixing ratio. In our opinion, the
effect of gas pressure can be explained as follows: an increase in
gas pressure at fixed CF4/Ar mixing ratio leads to an increase in
both density and flux of fluorine atoms on the etched surface,
but causes a decrease in ion flux and mean ion energy. As
a result, with increasing gas pressure, there is a tendency for
the chemical etch pathway to accelerate, but a worse condition
obtains for the ion stimulated desorption of reactive products,
probably resulting in a decreasing fraction of acceptable free
surface for chemical reaction [17]. Similar to the effect of the
gas mixing ratio discussed above, these two factors working in
opposite directions produce a non-monotonic behavior of the
etch rate.
Procedure (Condition): No data
Note: In this study, we carried out an investigation of the etching characteristics (etch rate, and selectivity to SiO2) of ZrO2
thin films in a CF4/Ar inductively coupled plasma (ICP) system. The maximum etch rate of 60.8 nm/min for ZrO2
thin films was obtained at a 20 % CF4/(CF4+Ar) gas mixing ratio. At the same time, the etch rate was measured as a
function of the etching parameter, namely ICP chamber pressure. X-ray photoelectron spectroscopy (XPS) analysis
showed efficient destruction of the oxide bonds by the ion bombardment, as well as an accumulation of low volatile
reaction products on the etched surface. Based on these data, the ion-assisted chemical reaction was proposed as
the main etch characteristics for the CF4-containing plasmas.
Reference: Han-Soo Kim, Jong-Chang Woo, Young-Hee Joo, and Chang-Il Kim, The Use of Inductively Coupled CF4/Ar Plasma to
Improve the Etch Rate of ZrO2 Thin Films, TRANSACTIONS ON ELECTRICAL AND ELECTRONIC MATERIALS, Vol. 14, No. 1, pp. 12-15, February 25, 2013.
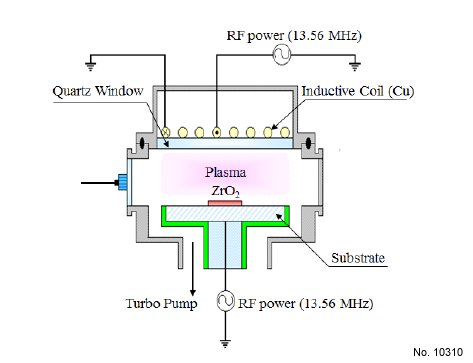
Figure 1: Schematic diagram of the inductively coupled plasma system
for ZrO2 thin film etching.
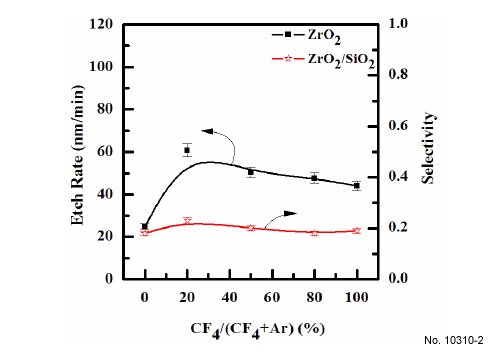
Figure 2: Etch rates of ZrO2 thin films as a function of the CF4/Ar gas
mixing ratio. The RF power was maintained at 600 W, the DC-bias
voltage was - 250 V, the process pressure was 15 mTorr, and the substrate
temperature was 45C.
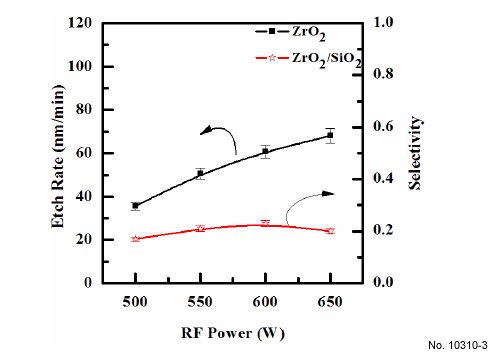
Figure 3: Etch rates of ZrO2 thin films as a function of the RF power. The
gas mixing was maintained at CF4/Ar(20:80%) plasma, the DC-bias
voltage was maintained at - 250 V, the process pressure was 15 mTorr,
and the substrate temperature was 45C.
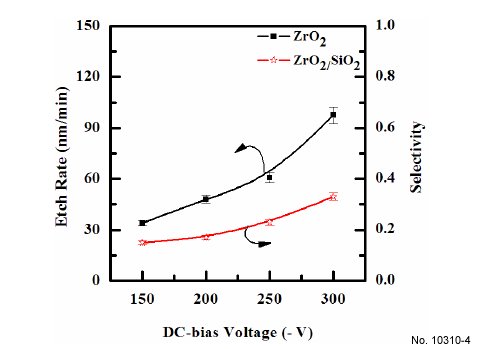
Figure 4: Etch rates of ZrO2 thin films as a function of the DC-bias voltage.
The gas mixing was maintained at CF4/Ar(20:80%) plasma, the
RF power was maintained at 600 W, the process pressure was 15
mTorr, and the substrate temperature was 45C.
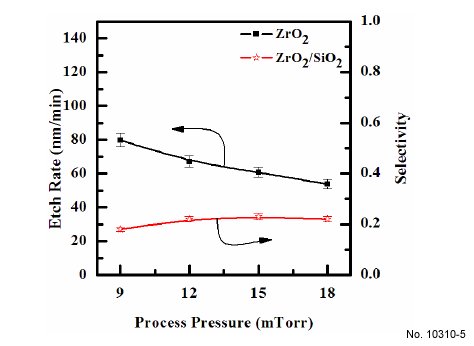
Figure 5: Etch rates of ZrO2 thin films as a function of the process pressure.
The gas mixing was maintained at CF4/Ar(20:80%) plasma, the
RF power was maintained at 600 W, the DC-bias voltage was - 250 V,
and the substrate temperature was 45C.