
Alphabetical Index
Browse by Elements
Keyword Search
Dry Etchants
Dry and Wet Etchants
Wet Etchants
Bulk Etchants
Layer Etchants
Nano Etchants
Single Crystal Etchants
Thin Film Etchants
Thin Foil Etchants
Wafer Etchants
Al Etchants
Cd Etchants
Ga Etchants
Ge Etchants
In Etchants
New Etchants
Other Etchants
Si Etchants
Zn Etchants
Help
Home
Sc2O3 Thin Film - Wet Etching
Material Name: Sc2O3
Recipe No.: 10315
Primary Chemical Element in Material: Sc
Sample Type: Thin film
Uses: Etching
Etchant Name: None
Etching Method: Wet etching
Etchant (Electrolyte) Composition: Three types of samples, each of 1 x 1 cm size, were prepared: 20 nm Sc, 3 nm and 5 nm Sc2O3/Sc
Sc2O3 (see FIG. 1(a), 1(b) and 1(c) for details). Scandium oxidizes very rapidly in ambient conditions,
creating a native oxide layer. To prevent Sc from oxidizing during deposition and sample handling,
the Si wafer substrates were covered with 5 nm of amorphous Si to protect the deposited Sc layer
from oxidizing via interaction with the native SiO2 layer on the Si wafer. After the Sc layer was
deposited, it was protected from oxidation with a 5 nm thick amorphous carbon (C) layer. It is known
that C can be etched from many surfaces with reactive hydrogen species, thus the C layer is
removed in-situ before Sn is deposited on top of this sample (see FIG. 1(a)). For the 5 nm Sc2O3/Sc
and 3 nm Sc2O3 samples, Sc was deposited directly on the Si wafer, which had a native oxide layer
of approximately 1.3 nm. To obtain a Sc2O3 top surface, these samples were exposed to ambient,
letting a native oxide layer to form. After deposition, the samples were characterized with angle
resolved (AR) XPS.
Tin deposition and etching takes place in an apparatus that has been described in detail elsewhere.1
Briefly, experiments are performed in two different manners: for oxidized samples, approximately
8 nm of Sn is evaporated onto the sample at a rate of 0.4 nm/min and, immediately afterwards, etched with hydrogen radicals (H.). For the metallic Sc sample, the C coating is first removed (determined
by ellipsometry) using H. etching, after which, 8 nm of Sn is deposited, followed by H. etching. In all
cases, the H. flux is generated by passing a molecular hydrogen flow (100 sccm) over a tungsten (W)
filament that is heated to 2000 C. The hydrogen radical flux that was measured to be 1017 at /s.cm2
at the sample surface, which was obtained from the carbon etching rate following the method used in
ref. 14. The filament was operated in 5 min cycles to avoid excessively heating of the sample surface.
In situ ellipsometry was used to monitor Sn deposition and etching. After Sn deposition and etching
experiments, the samples were analyzed ex situ with XPS, as well as SEM with energy selective
backscatter detector (SEM-ESB) and high efficiency secondary electron detector (SEM HE-SE2).
Procedure (Condition): No data
Note: The role of oxide on Sn adhesion to Sc surfaces was studied with in-situ ellipsometry,
X-ray photoelectron spectroscopy and secondary electron microscopy. Sn etching
with hydrogen radicals was performed on metallic Sc, metallic Sc with a native oxide,
and a fully oxidized Sc layer. The results show that Sn adsorbs rather weakly to a nonoxidized
Sc surface, and is etched relatively easily by atomic hydrogen. In contrast, the
presence of native oxide on Sc allows Sn to adsorb more strongly to the surface, slowing
the etching. Furthermore, thinner layers of scandium oxide result in weaker Sn adsorption,
indicating that the layer beneath the oxide plays a significant role in determining
the adsorption strength. Unexpectedly, for Sn on Sc2O3, and, to a lesser extent, for Sn
on Sc, the etch rate shows a variation over time, which is explained by surface restructuring,
temperature change, and hydrogen adsorption saturation.
Reference: M. Pachecka, et al., Tin etching from metallic and oxidized scandium thin films, AIP ADVANCES 7, 085107 (2017), pp. 085107-1 - 085107-8.
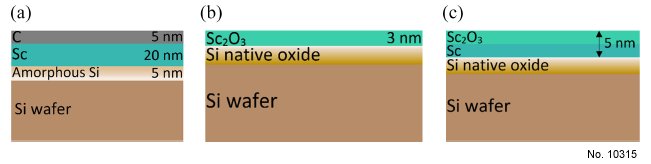
Figure 1: Composition of the samples used in this work: (a) 20 nm Sc, (b) 3 nm Sc2O3, (c) 5 nm Sc2O3/Sc.