
Alphabetical Index
Browse by Elements
Keyword Search
Dry Etchants
Dry and Wet Etchants
Wet Etchants
Bulk Etchants
Layer Etchants
Nano Etchants
Single Crystal Etchants
Thin Film Etchants
Thin Foil Etchants
Wafer Etchants
Al Etchants
Cd Etchants
Ga Etchants
Ge Etchants
In Etchants
New Etchants
Other Etchants
Si Etchants
Zn Etchants
Help
Home
Nb - Dry Etching
Material Name: Nb
Recipe No.: 10321
Primary Chemical Element in Material: Nb
Sample Type: Bulk
Uses: Etching
Etchant Name: None
Etching Method: Dry etching
Etchant (Electrolyte) Composition: The microwave cavity discharge system, capable of maintaining
discharges at pressures above 1 Torr. In the
microwave discharge, the high energy transfer efficiency
from the microwave electric field to the gas results in high
electron and high radical densities in the plasma. These
plasma conditions are more favorable for plasma etching
than for sputtering processes. Also, the higher gas
temperature in the plasma contributes to a higher rate of
chemical reactions and vaporization of Nb chlorides. The
Nb samples are placed in the quartz tube of a reaction
chamber. Low background gas pressure is achieved using
a system of mechanical and turbo molecular pumps, both
corrosive gas resistant. The gas in the reaction chamber
has high constant flow rates, so that reactive species lost
due to the chemical reaction can be replenished and
products of the chemical reaction removed away from the
sample. The gas flow control is achieved through flow
meters connected to a controller. For processes that
demand more than one reaction gas, a mixing chamber is
placed in front of the reaction chamber to facilitate a
better mixing of the gases. The experimental set-up is
connected to a spectrometer with the CCD camera for
emission spectroscopy measurements. Emission
spectroscopy is used as a process monitoring technique as
well as a tool to determine the reaction mechanism of the
plasma etching process.
We determined the etching rates from the difference in
sample mass before and after exposure to Ar/Cl2
discharge. From Table 1, one can see that the etching rate
increases with a higher amount of Cl2 in the gas mixture.
The dependence of the etching rate on the reactive gas
concentration is an indicator of the chemical etching
contribution to the Nb removal mechanism.
Procedure (Condition): No data
Note: The preparation of the cavity walls has been one of the
major challenges in the superconducting radio-frequency
(SRF) accelerator technology. Therefore, constant
research and development effort is devoted to develop
surface preparation processes that will improve roughness
and lower the level of impurities, like hydrogen or oxygen,
embedded in bulk Nb, having in the same time reasonable
etching rates. Plasma based surface modification provides
an excellent opportunity to achieve these goals. We
present Ar/Cl2 discharge treatment of bulk Nb where we
achieved etching rates comparable to the rates obtained
with the electropolishing method without introducing
impurities in Nb. The current experiments were
performed on disk shaped Nb samples, exposed to plasma
produced in a microwave discharge system. Surface
composition and topology measurements were carried out
before and after plasma treatment. Upon determining
optimal experimental conditions on disk shaped samples,
we will apply the same procedure on the single cell
cavities, pursuing improvement of their RF performance.
Reference: M. Raškovic, et al., PLASMA TREATMENT OF BULK Nb SURFACE IN
THE Ar/Cl2 DISCHARGE, Proceedings of SRF2007, Peking Univ., Beijing, China, TUP: Poster Session I, pp. 323-326.
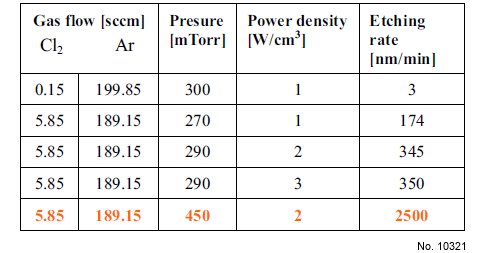
Figure 1: Experimental conditions and etching rates
obtained during plasma treatment of disk-shaped Nb
samples.