Alphabetical Index
Browse by Elements
Keyword Search
Dry Etchants
Dry and Wet Etchants
Wet Etchants
Bulk Etchants
Layer Etchants
Nano Etchants
Single Crystal Etchants
Thin Film Etchants
Thin Foil Etchants
Wafer Etchants
Al Etchants
Cd Etchants
Ga Etchants
Ge Etchants
In Etchants
New Etchants
Other Etchants
Si Etchants
Zn Etchants
Help
Home
GaAs - Wet Etching
Material Name: GaAs
Recipe No.: 10345
Primary Chemical Element in Material: Ga
Sample Type: Wafer
Uses: Etching
Etchant Name: None
Etching Method: Wet etching
Etchant (Electrolyte) Composition: Several wet chemical digital etching techniques were
attempted to determine one that provided consistent and
reproducible results. An important step in the etching
process is to completely separate the chemicals used for
each part of the digital etch step (oxidation step and oxide
etching step). That is, it is necessary to completely rinse the
H2O2 before applying the acid, and vice versa. Residue of
either H2O or acid on the GaAs wafer will mix with the
next application of acid or H2O2 and provide a normal acid:
H2O2 etchant that destroys the controlled, self-limiting digital
etching process. Because H2O2 and many acids contain
water, the use of deionized water (DI H2O) was selected to
provide the necessary rinsing between each step. To prevent
dilution of the chemical agents, an N2 blow dry is used
to remove any water remaining after rinsing.
A new method was developed to provide separately
fresh oxidizing and etching solutions to the sample during
each etch step to minimize chemical cross contamination.
Since contamination due to incomplete rinsing must be
eliminated to maintain the digital etching chemistry, a
photoresist spinner was modified to perform the digital
etching procedure. The modification of the photoresist
spinner consisted of replacing exposed metal parts (wafer
chuck, vacuum shaft, and spinner baseplate) with oxidizer
and acid resistant high density polypropylene machined
to original specifications. With the spinner, fresh oxidizing
and etching solutions can be sprayed from above onto the
sample during each etch step. The spinning motion removes
the applied solution quickly, and allows the application
of a continuously fresh stream of solution. DI H2O
rinsing of the wafer can also take place while spinning,
providing a continuously fresh water stream to remove the
oxidizing and etching solutions. In this manner, fresh solutions
of H2O2, acid, and DI H2O are applied to the sample
during the digital etch, thereby eliminating cross contamination
between H202 and the acid.
Process development has demonstrated that successful
digital etching requires the use of uncontaminated oxidizing
and etching solutions with complete rinsing between
the oxidizing and acid etching steps. Spin rinsing with
fresh DI H20 for 15 s at 1000 rpm was found to be sufficient
to remove both the oxidizing and etching solutions
from the wafer before applying the next solution in the digital etch process. However, it was determined that spinning
the wafer during applications of the oxidizing and
etching solutions was not necessary, as soaking the wafer
during these steps was determined to be sufficient. The
measured digital etch rates of this soak and spin rinsing
method are equivalent to those obtained using the continuous
spin technique. Soaking is desirable because it provides
a large reservoir of solution to perform the necessary
oxidation and etching without depleting the solution.
Because these experiments were performed manually, the
static chemical soak and spin rinsing technique was used
to maintain repeatability and reproducibility, as it was
difficult to insure complete coverage with manual spraying,
whereas a static flood was easy to accomplish and
maintain during the given soak times. The digital etching
experiments conducted in this work used a combined
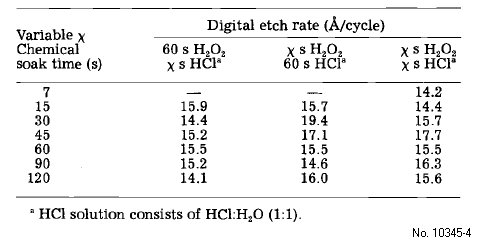
chemical soak and spin rinsing procedure as outlined in Table 1.
Procedure (Condition): No data
Note: A new room temperature wet chemical digital etching technique for GaAs is presented which uses hydrogen peroxide
and an acid in a two-step etching process to remove GaAs in approximately 15 A increments, In the first step, GaAs
is oxidized by 30% hydrogen peroxide to form an oxide layer that is diffusion limited to a thickness of 14 to 17 A for time
periods from 15 to 120 s. The second step removes this oxide layer with an acid that does not attack unoxidized GaAs.
These steps are repeated in succession until the desired etch depth is obtained. Experimental results are presented for this
digital etching technique demonstrating the etch rate and process invariability with respect to hydrogen peroxide and
acid exposure times.
Reference: Gregory C. DeSalvo, et al., Wet Chemical Digital Etching of GaAs at Room
Temperature, Journal of The Electrochemical Society, 143 (11), 1996, pp. 3652-3656.
Table 1: Experimental procedure used to perform a single wet chemical digital etching cyde.
Table 2: Digital etch rate dependence on acid or base using a 10 cycle digital etch step repetition with 1 min acid soaks and
1 min H202 soaks.
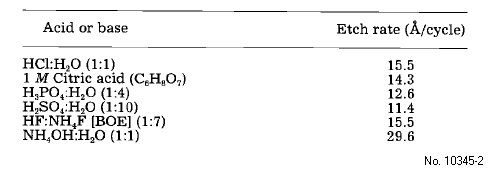
Table 3: Digital etch rate dependence on the number of digital etch cycles performed using 1 min HCI:H20 (l:1) soaks and 1 in H202 soaks.
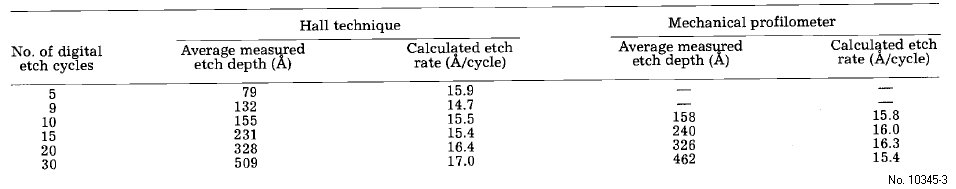
Table 4: Digital etch rate dependence on digital etch soak time
using a ten cycle digital etch repetition.