Alphabetical Index
Browse by Elements
Keyword Search
Dry Etchants
Dry and Wet Etchants
Wet Etchants
Bulk Etchants
Layer Etchants
Nano Etchants
Single Crystal Etchants
Thin Film Etchants
Thin Foil Etchants
Wafer Etchants
Al Etchants
Cd Etchants
Ga Etchants
Ge Etchants
In Etchants
New Etchants
Other Etchants
Si Etchants
Zn Etchants
Help
Home
AlSb - Wet Etching
Material Name: AlSb
Recipe No.: 8510
Primary Chemical Element in Material: Al
Sample Type: Substrate, bulk
Uses: Etching
Etchant Name: None
Etching Method: Wet etching
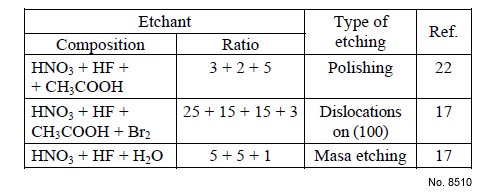
Etchant (Electrolyte) Composition: See the Table 1.
Procedure (Condition): No data
Note: Chemical etchants for GaSb and related compounds are given in Tables 1. Note that InSb, GaSb, and their solid solution require an oxidising agent. Most often HNO3 and H2O2 are used for this purpose. Only AlSb has sufficiently ionic character to not require oxidant and to react with HCl. Optimisation of solution composition is necessary for control of reaction method and products. For example, if the ratio of HNO3 to HCl is very large, insoluble antimony tri- or pentoxide forms while all other ratios yield soluble antimony chlorides. For strong acidic solutions addition of complexing agents such as tartaric or citric acid is necessary for prevent solution from total dissociation. Thus, ratios of HNO3 to organic acids must favour the organic acids. HNO3 is at the same time oxidising and etching agents because it oxidises III-V surfaces and etches III – V oxides (Ga2O3 and In2O3), simultaneously leaving the group V oxides (Sb2O3 and As2O3).
Reference: E. PAPIS-POLAKOWSKA, SURFACE TREATMENTS OF GaSb AND RELATED MATERIALS FOR THE PROCESSING OF MID-INFRARED SEMICONDUCTOR DEVICES, Electron Technology - Internet Journal 37/38 (2005/2006), 4, p. 1-34.
Table 1: Chemical etchants for AlSb.