
Alphabetical Index
Chemical Composition
Keyword Search
Named Inclusions
Steel Index
Exogenous Inclusions
Indigenous Inclusions
Macro Inclusions
Micro Inclusions
Nano Inclusions
Iron Oxide Inclusions
Nitride Inclusions
Oxide Inclusions
Phosphide Inclusions
Silicate Inclusions
Spinel Inclusions
Sulfide Inclusions
Refractory Inclusions
Slag Inclusions
Figure Browser
Help
Contact Us
Home
The iron sulfide coexisted with silicon oxide with different morphologies in sample H13L
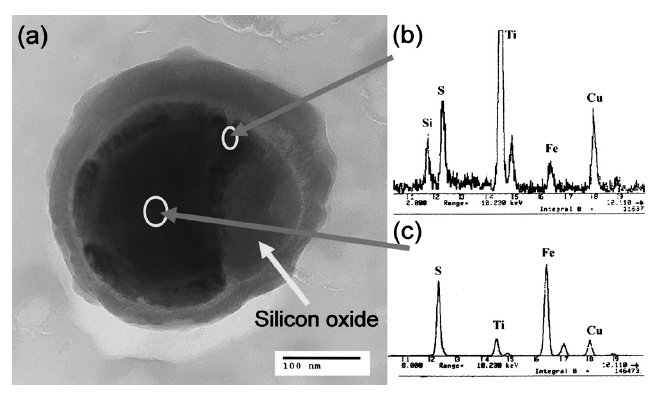
Figure 1: The iron sulfide coexisted with silicon oxide in sample H13L (Ti grid). (a) TEM image; STEM-EDS spectra from the edge (b) and the center (c) of sulfide. Scale bar: 100 nm.
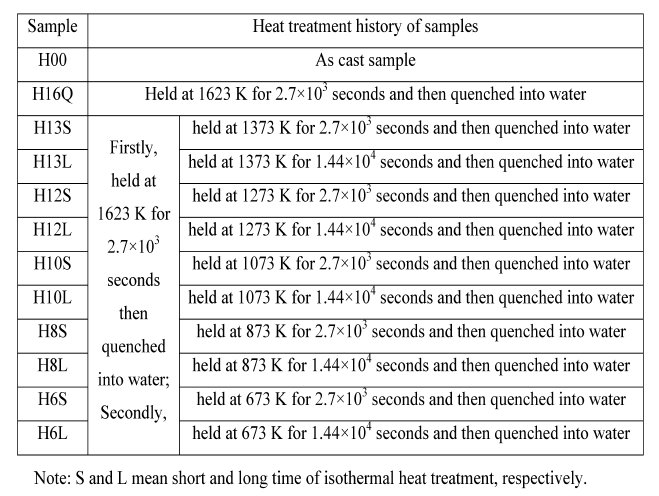
Table 1: The detail heat treatment history of samples.
Inclusion name: Iron sulfide and silicon oxide
Record No.: 869
Inclusion formula: FeS, SiO2
Inclusion type (Macro/Micro/Nano): Nano
Inclusion type (Exogenous/Indigenous): Indigenous
Inclusion classification: Sulfide, oxide
Inclusion composition in weight %: No data
Sample: Steel
Steel composition in weight %: 0.005% C, 0.25% Cu, 0.024% S, 0.0080% P, 0.0012% N.
Note: Copper and sulfur are typical residual elements or impurity elements in steel. Sufficient removal of them during steelmaking process is difficult for copper and costly for sulfur. Utilization of copper and sulfur in steel, especially in steel scrap, has been an important issue for a long period for metallurgists. Copper and sulfur may combine to form a copper sulfide, which may provide a prospect to avoid the detrimental effects of copper and sulfur in steel. Unfortunately the formation mechanism of a copper sulfide
in steel has not been completely clarified so far. In the present paper, solution treatment of samples containing copper and sulfur are firstly performed at 1623 K for 2.7 x exp10(3) s followed by quenching into water. The samples are then isothermally heat-treated at 673 K, 873 K, 1073 K, 1273 K and 1373 K for different time followed by quenching into water again. The size, morphology, constituent and crystallography of sulfide precipitates in these samples are investigated by SEM and TEM equipped with EDS. Fine copper sulfides (less than 100 nm) are observed to co-exist with silicon oxide in samples even isothermally heattreated at 1373 K for 1.44 x exp 10(4)s. Film-like copper sulfides are generally observed to co-exist with iron sulfide in all samples; Plate-like copper sulfides are observed especially in sample isothermally heat-treated at 1073 K for 1.44 x exp10(4) s. The formation mechanisms of these copper sulfides have been discussed in detail.
Although it is not clear that the little iron accompanying with the CuxS coexisted with silicon oxide is solid solution one in CuxS or just an attachment on it, the little copper sulfide accompanying with iron sulfide seems just a shell/film attached on the iron sulfide, as shown in Fig. 1. The STEM-EDS spectrums show the Cu concentration at the edge of
the iron sulfide particle is quite higher than that of the interior part, which means that the iron sulfide is covered by a copper sulfide shell/film.
Additional links: Not shown in this demo version.
Reference: Not shown in this demo version.