
Alphabetical Index
Chemical Composition
Keyword Search
Named Inclusions
Steel Index
Exogenous Inclusions
Indigenous Inclusions
Macro Inclusions
Micro Inclusions
Nano Inclusions
Iron Oxide Inclusions
Nitride Inclusions
Oxide Inclusions
Phosphide Inclusions
Silicate Inclusions
Spinel Inclusions
Sulfide Inclusions
Refractory Inclusions
Slag Inclusions
Figure Browser
Help
Contact Us
Home
Hematite
Chemical formula: Fe2O3
Modifications: In addition to alpha-Fe2O3 hematite , the metastable gamma-Fe2O3 exist. The later is formed by oxidation of magnetite bellow 350oC and it has a defect spinel type lattice. At higher temperatures the Fe2O3 phase loses oxygen and Fe3O4 is formed. The oxygen pressure over Fe2O3 is 0.5 mm Hg at 1150oC, increasing to 1 atm at 1457oC. Above this temperature, therefore no formation of Fe2O3 occurs if steel is oxidized at atmospheric pressure.
Melting point: 1730oC
Density: 5.24 g/cm3
Microhardnes: No data
Hardness (Moh's): 6.5
Luminescence: Non-fluorescent
Colour in air, bright field reflection: Light grey, whitish
Colour, polarized reflection: No data
Shape: No data
Crystal system: Tetragonal
Cell dimensions: a=5.028 A, c=13.73 A
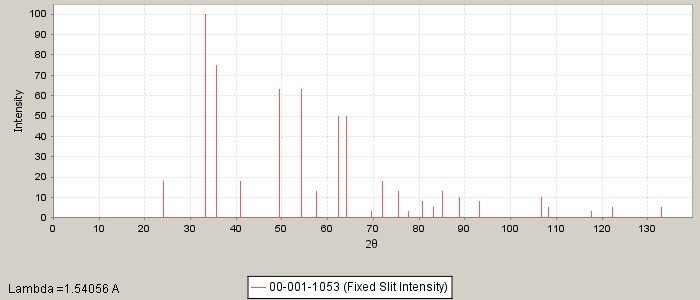
PDF number: 00-001-1053
ICSD number: 064599
Note: Hematite and Cr2O3 are reported to have a complete solid solubility range at all temperatures above 20oC. Hematite also dissolves considerable amounts of corndum. The solid solubality limit depends on temperature and oxygen pressure. Hematite is only iron oxide which shows a marked anisotropy and therefore easily recognizable in the microscope. Hematite is that iron oxide which is richest in oxygen , and therefore forms the outermost scale when iron and steel are oxidized. Fe2O3 layer is usually thin and disappears completely above 1457oC at atmospheric pressure.