
Alphabetical Index
Chemical Composition
Keyword Search
Named Inclusions
Steel Index
Exogenous Inclusions
Indigenous Inclusions
Macro Inclusions
Micro Inclusions
Nano Inclusions
Iron Oxide Inclusions
Nitride Inclusions
Oxide Inclusions
Phosphide Inclusions
Silicate Inclusions
Spinel Inclusions
Sulfide Inclusions
Refractory Inclusions
Slag Inclusions
Figure Browser
Help
Contact Us
Home
Iron Sulfide
Chemical formula: FeS
Modifications: Several modifications of FeS exist, crystallographically closly related but differing slightly in composition. The most common modifications, pyrite (FeS2) and marcasite (FeS2).
Melting point: 1190oC
Density: 4.77 g/cm3
Microhardnes: FeS is reported to be harder than MnS (Kiessling and Lange, 1964)
Hardness (Moh's): 3.5-4
Colour: FeS has a yellow ochre colour in the optical microscope. It is anisotropic and therefore is active in polarized light, whereas the cubic MnS is inactive.
Colour in air, bright field reflection: Dirty yellow to light brown (FeS to Fe11S12)
Colour, polarized reflection: Coloured
Shape: Deformable
Crystal system: Hexagonal
Cell dimensions: a=3.43 A, c=5.68 A
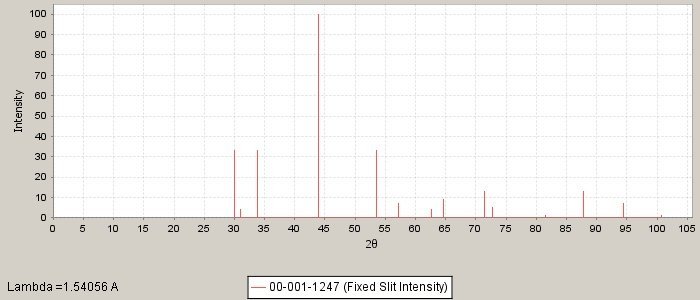
PDF number: 00-001-1247
ICSD number: 035005
Note: Fe(1-x)S with lower sulphur content (x=0) has a hexagonal lattice and is superstructure of the NiAs lattice. This phase as a mineral has the name troilite (FeS). For higher sulphur content (increasing x-values) the crystal structure of Fe(1-x)S cahnges to the more simple basic structure of NiAs, and the pyrrhotite minerals usuallyhave this crystal strcture. FeS inclusions may have a Cr content of up to 20%, They have also been found with Ti and V in solid solution, whereas the solid solubility for Mn is reported to be low. However, they found a solid solubility of about 7% of Mn in FeS. The solid solubility of C and O in FeS is low. No carbosulfides or oxysulfides of iron have been reported, but two-phase inclusions with FeS and FeO or with FeS and Fe-silicate are common. The formation of hot-shotness: Sulphur is soluble in liquid iron but the solubility us very low in the solid iron phase. When iron solidifies, sulphur therefore precipitates as FeS, either as primary FeS dendrites or as eutectic constituent with iron. This eutectic has a low melting point 988oC and the melting point is decreased by the influence of oxygen.For instance the FeO-FeS eutectic has a melting point about 988oC. Sulphur therefore segregates to those part of the ingot which are the last to solidify and the grain boundaries of these parts will be rich in FeS. The sulfide phase may either from primary FeS or be present in different eutectics. If the ingot is reheated to temperatures in the range of 980-1200oC a liquid phase my be formed in the grain boundaries. This may cause cracking of the iron, starting in the grain boundaries, if the iron is plastically deformed. This phenomenon is called hot-shortness.
In modern steels formation of FeS is avoided by Mn additions and FeS is not a common phase in steels.