
Alphabetical Index
Browse by Elements
Keyword Search
ASTM Electrolytes
Macro Etchants
Micro Etchants
Named Etchants
New Etchants
Al and Al Alloys
Cu and Cu Alloys
Fe and Fe Alloys
Ni and Ni Alloys
Carbide Etchants
Fluoride Etchants
Nitride Etchants
Other Etchants
Oxide Etchants
Phosphide Etchants
Single Crystal Etchants
Thin Film Etchants
Wafer Etchants
Help
Home
Electrolytic Polishing
Even with the most careful mechanical polishing, some disturbed metal
however small the amount, will remain after preparation of a metallographic
specimen. This is no problem if the specimen is to be etched for structural
investigation, because etching is usually sufficient to remove the slight
layer of disturbed metal. If the specimen is to be examined in the as-
polished condition, however, or if no surface disturbance can be tolerated,
either electrolytic polishing or chemical polishing is preferred.
Note: Image by courtesy of Oxford Instruments.
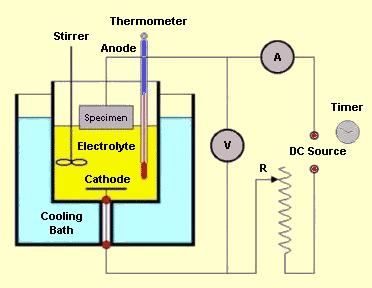
Electrolytic preparation uses an electrolytic reaction cell containing a liquid electrolyte with two electrodes: an anode and cathode. The sample to be polished/etched forms the anode. The electrodes are connected to an external power supply and voltage applied to cause reaction within the cell.
In electrolytic polishing (elektropolishing), the specimen is the anode
in an electrolytic cell. Direct current from an external cell is applied to
the electrolytic cell under specific conditions, and anodic dissolution
results in a leveling and brightening of the specimen surface. The most
widely accepted theory postulated to explain the leveling action concerns
the layer of viscous material (usually visible) that is present on the
specimen surface during electropolishing. This layer (termed the anolyte
layer) is composed of products that result from the reaction between the
metal and the electrolyte, and is, according to the theory, necessary for
proper electropolishing. At areas of elevation on the specimen surface,
this anolyte layer is thinner and offers less resistance to the flow of
current than areas of depression. The resulting higher current density
at elevated areas causes the metal in those areas to dissolve more
rapidly than metal in depressed areas, and thus levels the surface of
the specimen. The brightening of the anodic surface is atributed to the
formation of a thin film that follows contours of the surface and to
uniform attack of this film by the electrolyte.
Note: Image by courtesy of Oxford Instruments.
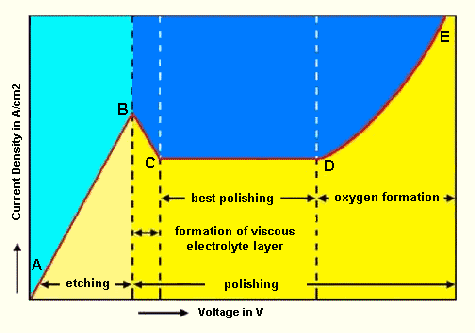
Shown above is the characteristic curve for an electrolytic cell. This curve is dependant on the electrolyte used and will vary for different electrolytes. Control of the voltage and current density at the anode, plus electrolyte composition, temperature and agitation are all critical in achieving the desired etching/polishing characteristics. Establishing adequate control of these parameters can be difficult and further, many of the electrolytes are hazardous or even explosive. In the case of the latter, temperature control is critical. Do not attempt electro polishing or etching without the necessary experience and safety measures in place.
Electropolishing does not disturb any metal on the specimen surface
and therefore is ideally suited for the metallographic preparation of
soft metals, most single-phase alloys, and alloys that work harden readily.
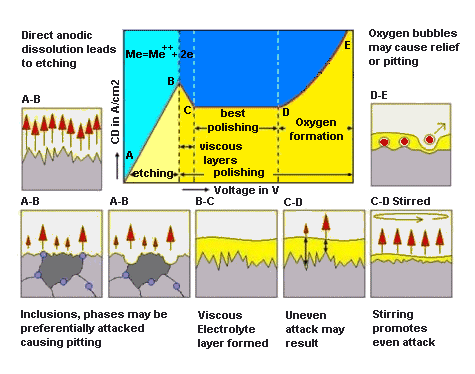
The disadvantages of electropolishing include preferential attack in
multiphase alloys caused by differences in electrical potential between
phases, and chemical attack of nonmetalic inclusions by the electrolyte.
Proper choice of electrolyte and operating conditions will minimize
these disadvantages.
Note: Image by courtesy of Oxford Instruments.
Factors controlling etching/polishing characteristics include:
Electrolyte composition
Electrolyte temperature
Electrolyte stirring
Area to be polished/etched (current density)
Voltage
Advantages - Disadvantages of Electrolytic Etching or Polishing:
Fast
Can be reproducible
No mechanical deformation
Can be automated
Can produce excellent surfaces for examination
Conductive specimens only
Not all alloys can be polished
Preferential attack or pitting can occur
No edge retention
Limited polishing area
Limited scratch/material removal
Hazardous Electrolytes
Temperature control vital
Establishing correct conditions can be difficult