
Alphabetical Index
Chemical Composition of Steels
Keyword Search
Steel Names
Alloyed Steels
Carbon Steels
Cast Irons
Chromium Steels
Cold Work Tool Steels
Creep Resistant Steels
Hot Work Tool Steels
Molybdenum Steels
PM steels
Stainless Steels
Structural Steels
Tool Steels
Vanadium Steels
White Cast Irons
M2C Carbides
M3C Carbides
M7C3 Carbides
M23C6 Carbides
MC Carbides
Light Microscopy
EDS/WDS Microanalysis
Scanning Electron Microscopy
Transmission Electron Microscopy
X-Ray Diffraction
Help
Contact Us
Home
M23C6 carbides in 18Cr–12Ni steel
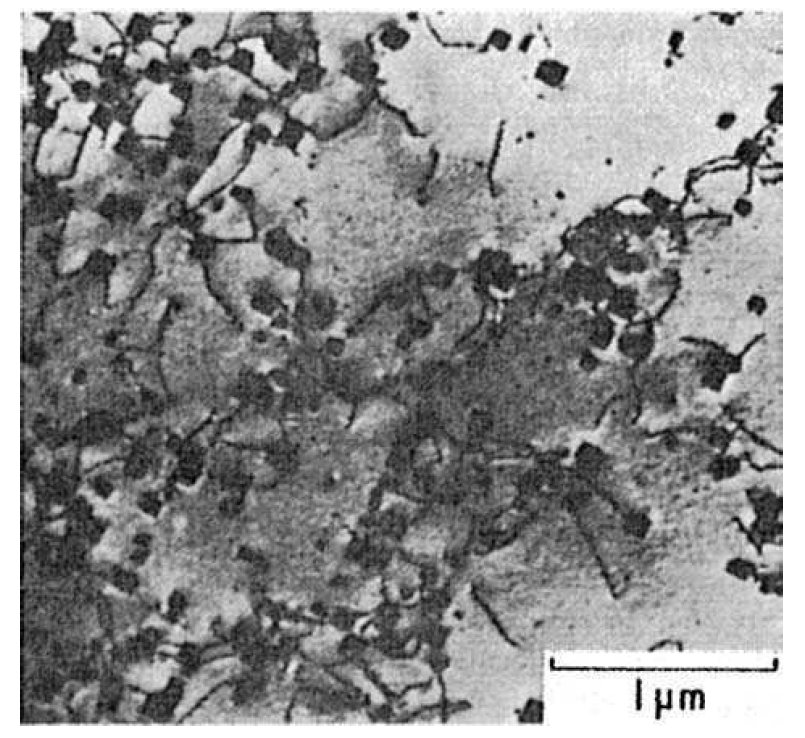
Figure 1: Precipitation of M23C6 on dislocations in a 18Cr–12Ni steel after 80 h at 700 C
under stress (courtesy of Sully). Thin-foil electron micrograph. Scale bar: 1 µm.
Carbide name: M23C6
Record No.: 1167
Carbide formula: M23C6
Carbide type: M23C6
Carbide composition in weight %: No data
Image type: No data
Steel name: 18Cr–12Ni
Mat.No. (Wr.Nr.) designation: No data
DIN designation: No data
AISI/SAE/ASTM designation: No data
Other designation: No data
Steel group: Stainless steels
Steel composition in weight %: 18Cr–12Ni
Heat treatment/condition: No data
Note: Simple austenitic steels usually contain between 18 and 30wt% chromium, 8
to 20wt% nickel and between 0.03 and 0.1 wt% carbon. The solubility limit
of carbon is about 0.05 wt% at 800 C, rising to 0.5 wt% at 1100 C. Therefore,
solution treatment between 1050 C and 1150 C will take all of the carbon into
solution and rapid cooling from this temperature range will give a supersaturated
austenite solid solution at room temperature. However, slow cooling or
reheating within the range 550–800 C will lead to the rejection of carbon from
solution, usually as the chromium-rich carbide, Cr23C6, even when the carbon
content of the steel is very low (<0.05 wt%).
This carbide nucleates preferentially at the austenitic grain boundaries as
faceted particles or often as complex dendritic arrays. While such
precipitation can have an adverse effect on mechanical properties, in particular
low-temperature ductility, the most significant result is the depletion of the
regions adjacent to the grain boundaries with respect to chromium.This has been
revealed directly by microprobe analysis.The surface film in these regions is thus
depleted in chromium and as a result the steel is more prone to corrosive attack.
Consequently, a classic form of intergranular corrosion is experienced which, in
severe cases, can lead to disintegration of the steel.This type of corrosion is also experienced in martensitic chromium steels, e.g. 12 wt% Cr steel, in which grain
boundary precipitation of Cr23C6 occurs as well.
Cr23C6 also precipitates within the austenite grains, particularly at higher
supersaturations, on dislocations and on solute atom/vacancy clusters. The lattice parameter of M23C6 is approximately three times that of austenite,
so the electron diffraction patterns are readily identified. The particles usually
develop a polyhedral habit, but occasionally in steels deformed at elevated temperatures
a more regular cubic morphology is displayed (Fig. 1). As the critical
temperature range for chromium carbide nucleation and growth is between
500 C and 850 C, any process which allows the steel to pass slowly through
this temperature range will render it sensitive to intergranular corrosion in service.
Welding, in particular, provides these conditions in the heat affected zone
(HAZ) leading to localized attack in certain chemical media. It is, therefore,
important to have information about the reaction kinetics for the formation of
Cr23C6. Being a typical nucleation and growth process, the time–temperature–transformation (TTT) curve is typically C-shaped with the nose at about 750 C.
Links: No data
Reference: Not shown in this demo version.