
Alphabetical Index
Chemical Composition of Steels
Keyword Search
Steel Names
Alloyed Steels
Carbon Steels
Cast Irons
Chromium Steels
Cold Work Tool Steels
Creep Resistant Steels
Hot Work Tool Steels
Molybdenum Steels
PM steels
Stainless Steels
Structural Steels
Tool Steels
Vanadium Steels
White Cast Irons
M2C Carbides
M3C Carbides
M7C3 Carbides
M23C6 Carbides
MC Carbides
Light Microscopy
EDS/WDS Microanalysis
Scanning Electron Microscopy
Transmission Electron Microscopy
X-Ray Diffraction
Help
Contact Us
Home
Steels with high chromium and/or nickel contents
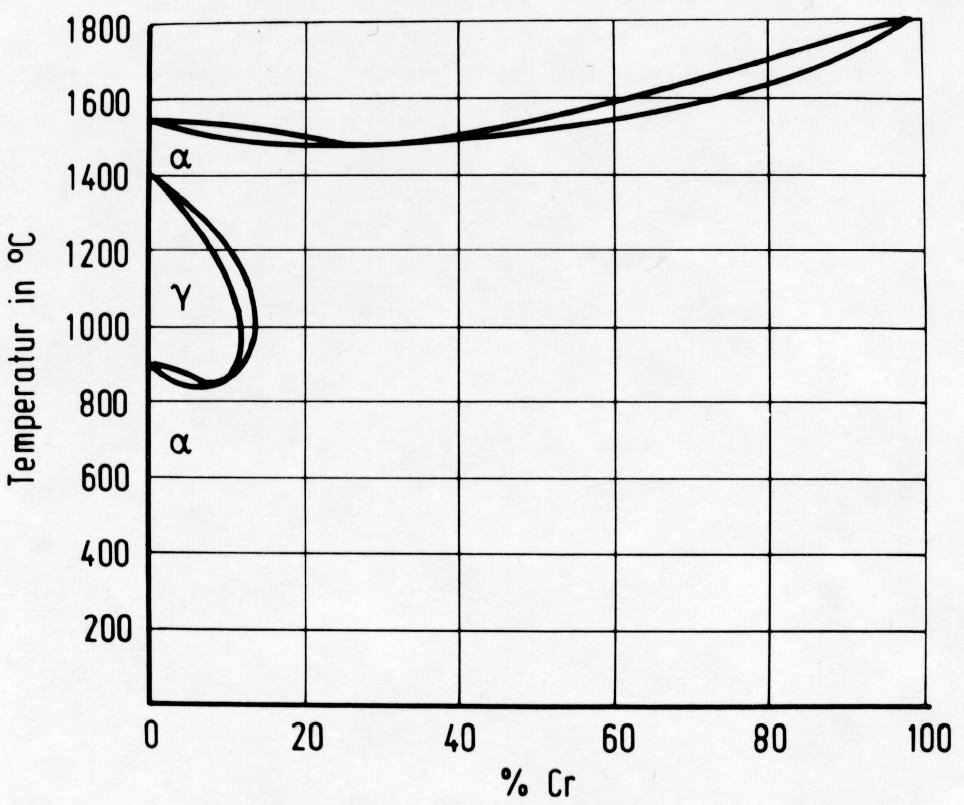
Figure 1: Binary Fe-Cr system with bounded gamma-field.
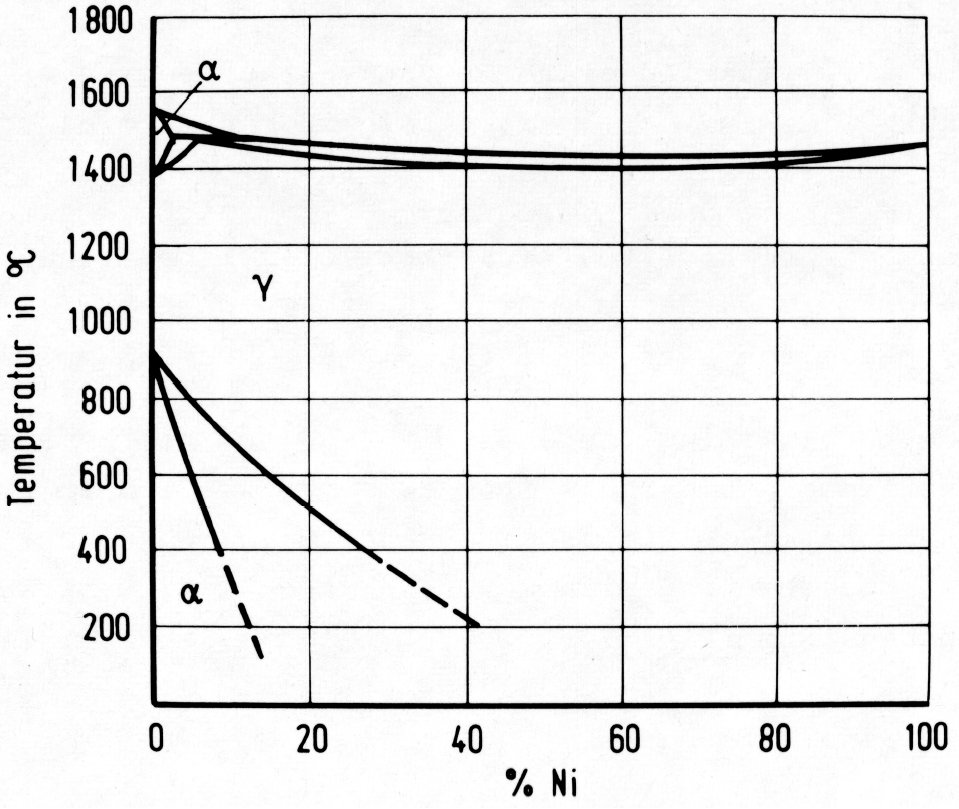
Figure 2: Binary Fe-Ni system with open gamma-field.
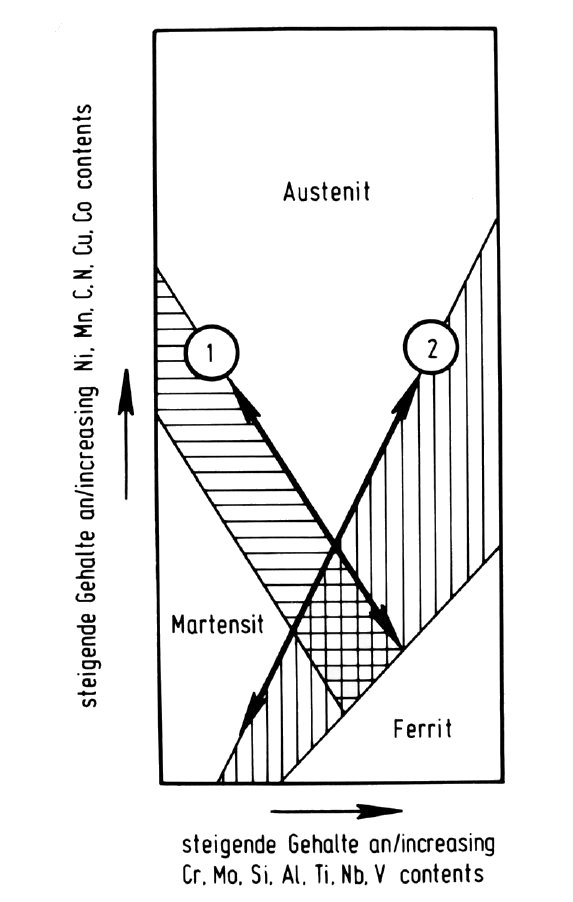
Figure 3: Microstructure and transformation of high-alloy chromium-nickel-base steels.
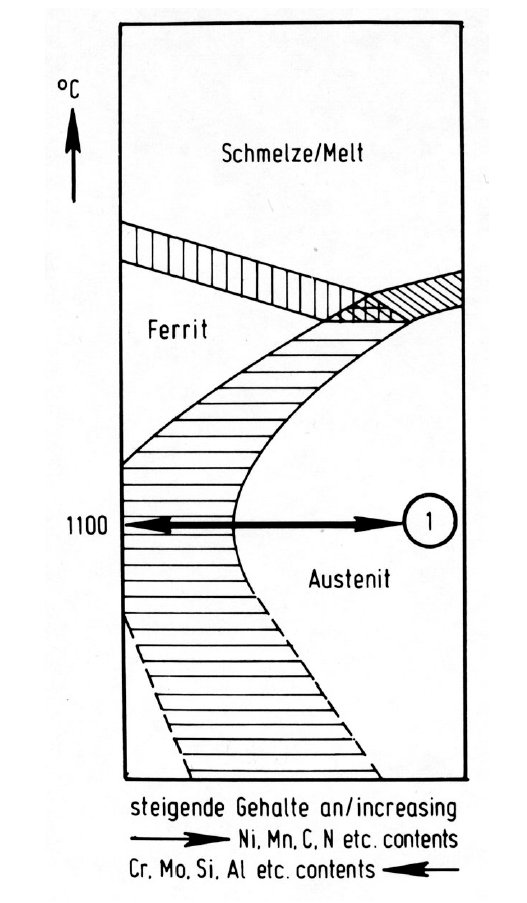
Figure 4: Microstructure and transformation of high-alloy chromium-nickel-base steels.
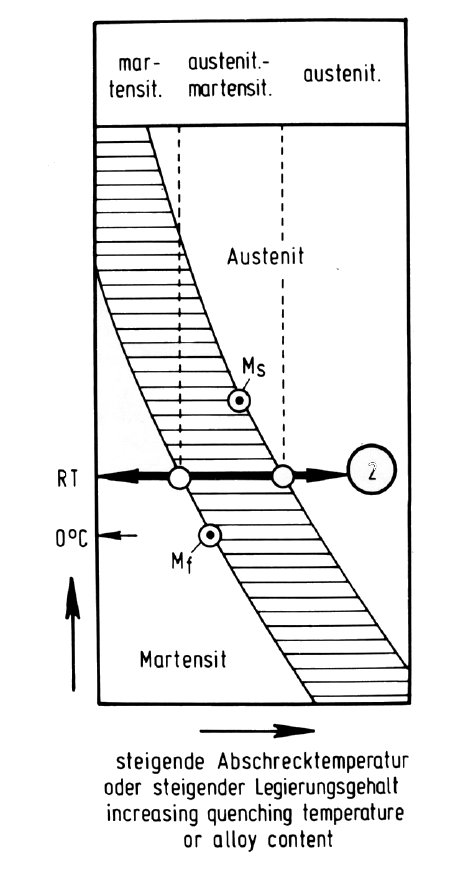
Figure 5: Microstructure and transformation of high-alloy chromium-nickel-base steels.
Carbide name: No data
Record No.: 1492
Carbide formula: No data
Carbide type: No data
Carbide composition in weight %: No data
Image type: No data
Steel name: Steel
Mat.No. (Wr.Nr.) designation: No data
DIN designation: No data
AISI/SAE/ASTM designation: No data
Other designation: No data
Steel group: Steels with high chromium and/or nickel contents
Steel composition in weight %: No data
Heat treatment/condition: See the text
Note: Steels with high chromium and/or nickel contents may be divided into three main groups according to the crystal structure of the matrix and the effect of heat treatments on it:
a) ferritic steels,
b) austenitic steels,
c) martensitic steels.
Transitions between these main groups lead to the sub-groups:
d) ferritic-austenitic steels,
e) austenitic-martensitic steels.
The differences in microstructure are due to the effect of the alloy elements on the alpha-gamma- transformation of iron.
Chromium causes a "constriction" on the gamma-region, as shown in Fig. 1. The range of existence of the austenite is thus reduced, and chromium is termed a "ferrite former". Other ferrite formers in alloying technology are above all molybdenum, silicon and aluminium. In contrast to chromium, the other ferrite formers, e.g. molybdenum, have only a limited solubility in the ferrite. They form intermetallic compounds with iron, e.g. Fe2Mo, which reduce the range of existence of the homogeneous ferrite with respect to Fig. 1.
Unlike chromium, nickel favours the formation of austenite by lowering the A3 temperature and raising the A4 temperature as shown in Fig. 2. The range of existence of the austenite is extended. This phenomenon is known as an "open gamma field" or "austenite stabilization". The elements having this effect are known as "austenite formers". The principal austenite formers, apart from nickel, among the main alloying elements of steels, are manganese, copper and cobalt, and also carbon and nitrogen.
In the case of these elements also, a distinction must be made between those binary systems in which as in the case of iron-nickel, the gamma region is limited by the formation of solid solutions (Fig. 2), and those in which it is limited by heterogeneous precipitation. For the latter the main elements responsible are interstitial carbon and nitrogen, which form carbides and nitrides with the iron.
In the multi-component alloy "steel" the interaction between the elements present leads to displacement of the phase regions, with respect to the binary iron alloys, and to the formation of new compounds, such as the carbides M23C6 (e.g. Fe21Mo2C6 ) and M6C (e.g. Fe3W3C) or the intermetallic compounds xsi-phase (e.g. Cr12Fe36Mo10 ) and G-phase (e.g. Ni13Ti8Si6).
For the multi-purpose group of the austenitic chromium-nickel steels the fact that chromium amplifies the austenite stabilizing effect of nickel is of decisive importance. This was the origin, beginning with the now classical austenitic 18 Cr/8 Ni steel, of the development of the stainless, heat-resisting steels and also the non-magnetic materials with low-temperature toughness and high-temperature strength.
The schematic diagrams in Fig. 3 to Fig. 51) serve to explain the above-mentioned microstructures of the high-alloy, chromium-nickel basis steels. Fig. 3, according to the principal of the Schäffler diagram, shows the microstructure as a function of the sum of the ferrite-forming elements chromium, molybdenum, silicon, aluminium etc. as abscissa and the austenite-forming elements nickel, manganese, carbon, nitrogen etc. as ordinate. The adjustment of the ferrite-forming and austenite-forming elements in the direction of the double arrow labelled "1" regulates the austenite or austenite-ferrite microstructure which forms after solution treatment at about 1100 °C, as shown in Fig. 4 (groups a, b and d).
In contrast to the opposing effect of the ferrite-forming and austenite-forming elements on the austenite/ferrite ratio, the stability of the austenite with respect to martensite formation on cooling depends only on the total amount of both types of element with respect to the iron content, as shown by the double arrow labelled "2" in Fig. 3. With decreasing alloy content the Ms temperature rises accordingly as shown in Fig. 5. If the formation of martensite is more or less complete at room temperature, the steel is called martensitic (group c). If it begins at room temperature or below, however, the steel is called austenitic (group b). Between these two extremes, the austenitic-martensitic steels form the sub-group e.
Links: No data
Reference: Atlas of Precipitates in Steels, Verlag Stahleisen GmbH, Germany, December 1983, pp. 404-411.