
Alphabetical Index
Chemical Composition of Steels
Keyword Search
Steel Names
Alloyed Steels
Carbon Steels
Cast Irons
Chromium Steels
Cold Work Tool Steels
Creep Resistant Steels
Hot Work Tool Steels
Molybdenum Steels
PM steels
Stainless Steels
Structural Steels
Tool Steels
Vanadium Steels
White Cast Irons
M2C Carbides
M3C Carbides
M7C3 Carbides
M23C6 Carbides
MC Carbides
Light Microscopy
EDS/WDS Microanalysis
Scanning Electron Microscopy
Transmission Electron Microscopy
X-Ray Diffraction
Help
Contact Us
Home
Carbides in Vanadis PM steel
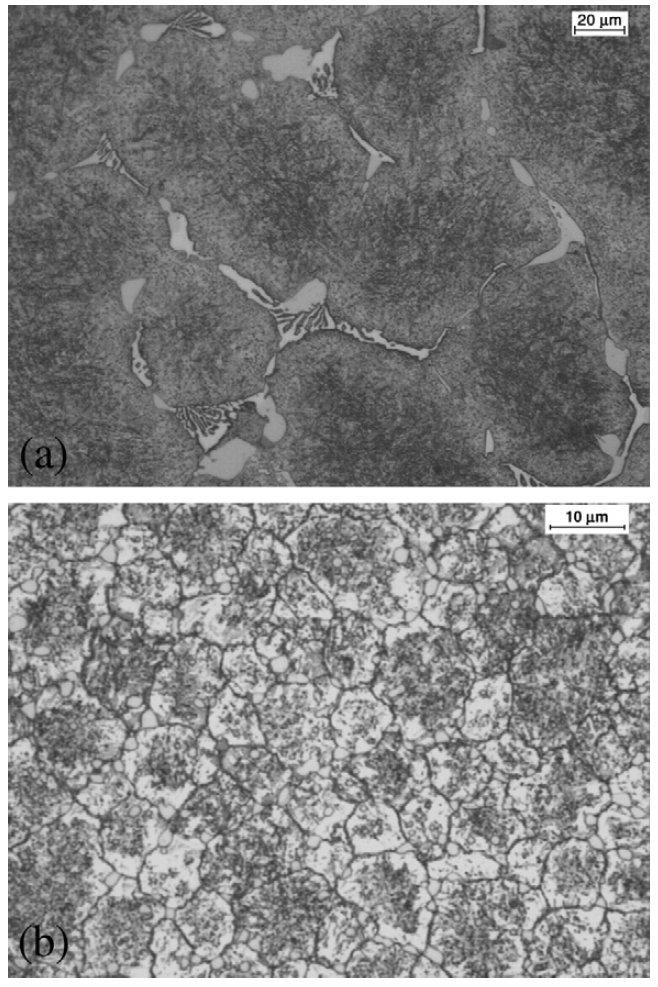
Figure 1: Optical micrographs of V4 steel: (a) as-cast,(b) as-sprayed, Scale bars: 20, 10 µm.
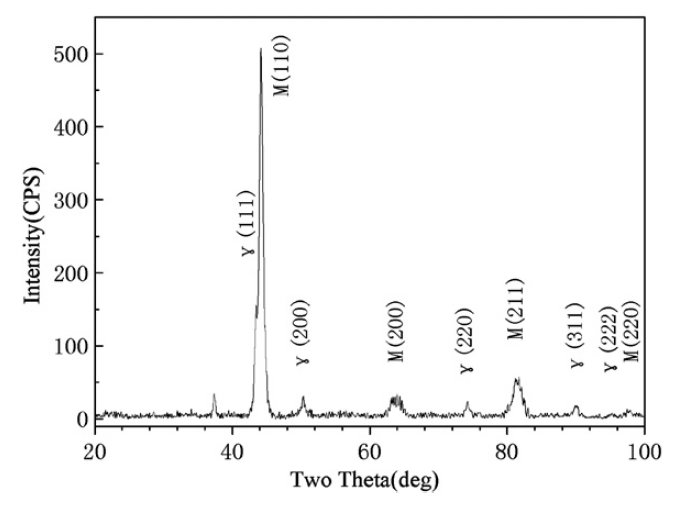
Figure 2: X-ray diffraction spectrum of the as-sprayed V4 steel.
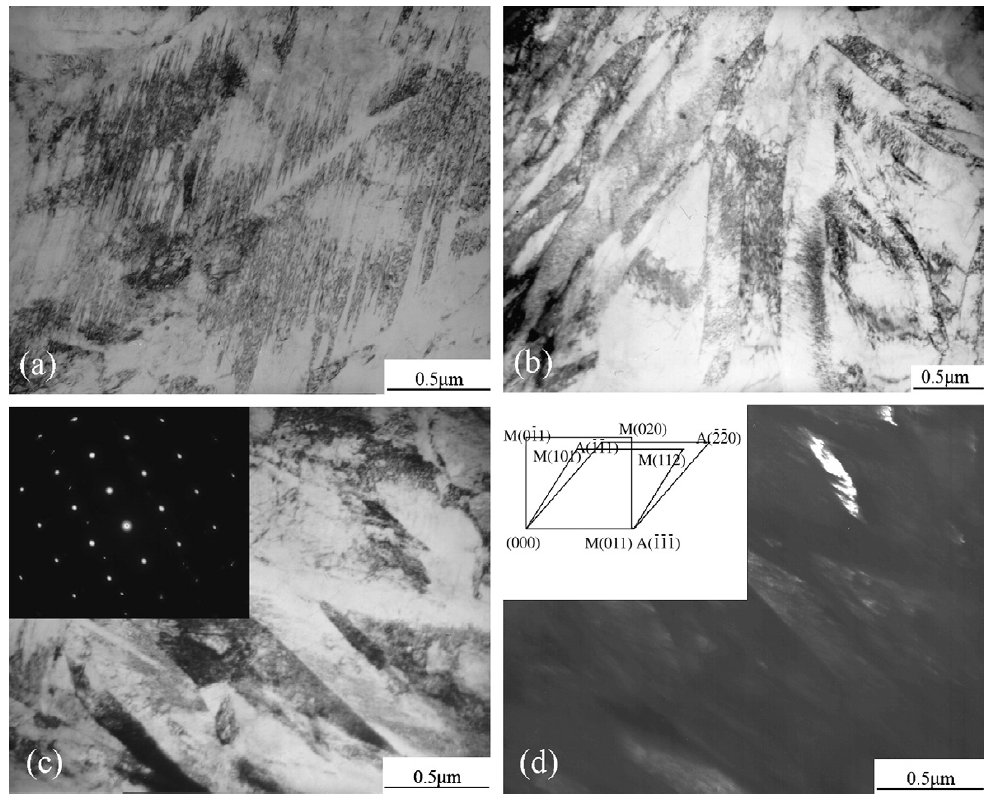
Figure 3: TEM micrographs: (a) twined martensite, (b) lath martensite, (c) retained austenite (bright field) and its diffraction
patterns, (d) retained austenite (dark field) and standardization of diffraction patterns. Scale bars: 0.5 µm.
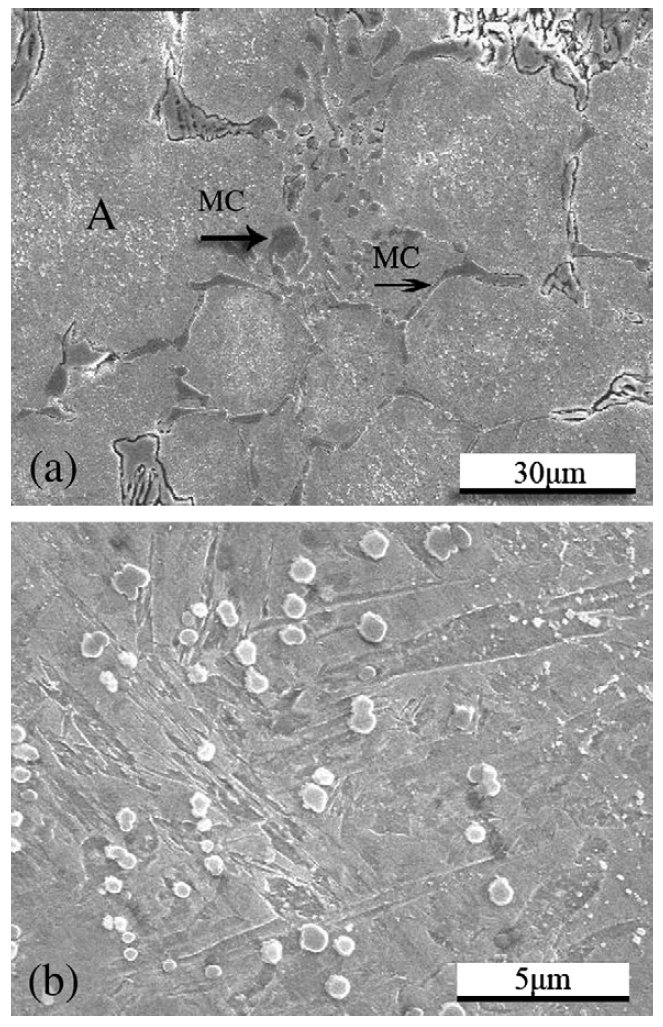
Figure 4: SEM micrographs of the as-cast V4 steel. Scale bars: 30, 5 µm.
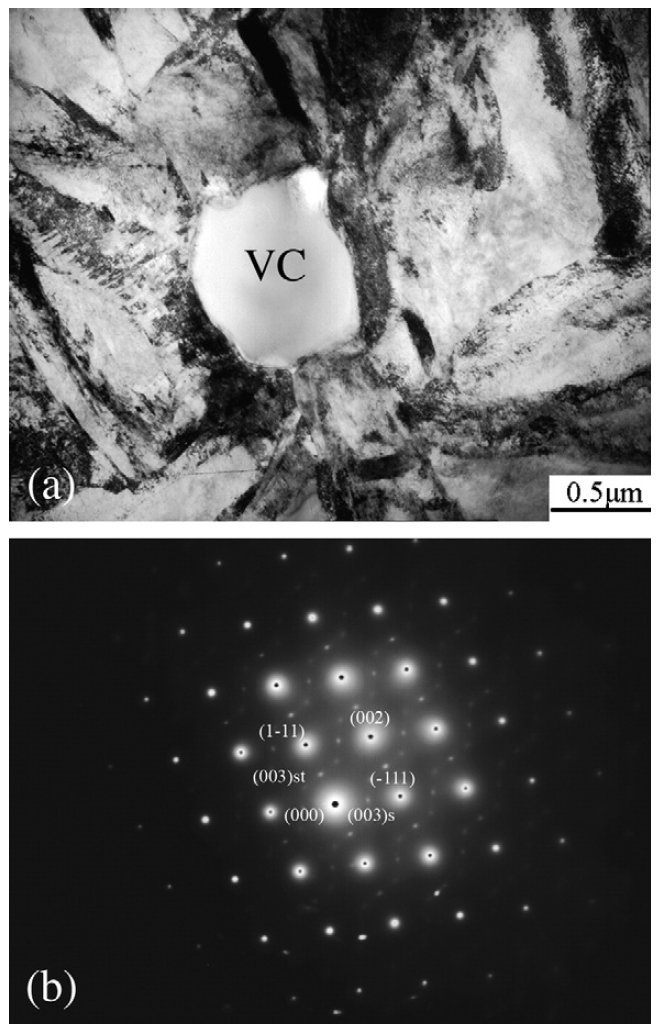
Figure 5: (a) TEM micrograph of VC in the as-sprayed V4 steel, (b) selected area diffraction patterns in [011] zone axis of VC. Scale bar: 0.5 µm.
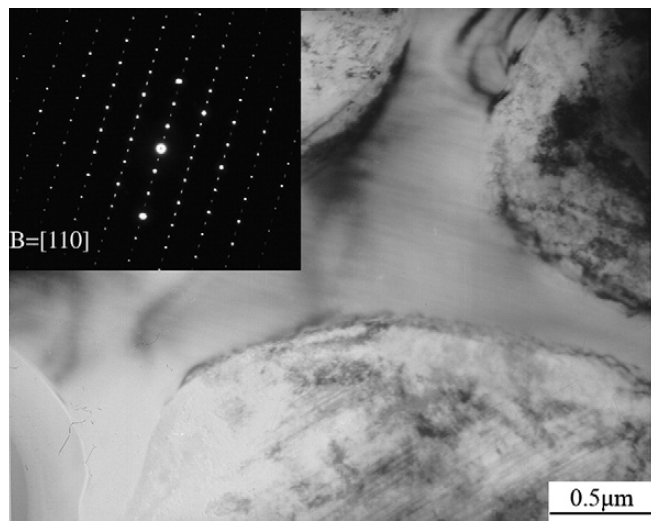
Figure 6: TEM micrograph of the M7C3 carbide in the as-sprayed V4 steel and its selected area diffraction patterns. Scale bar: 0.5 µm.
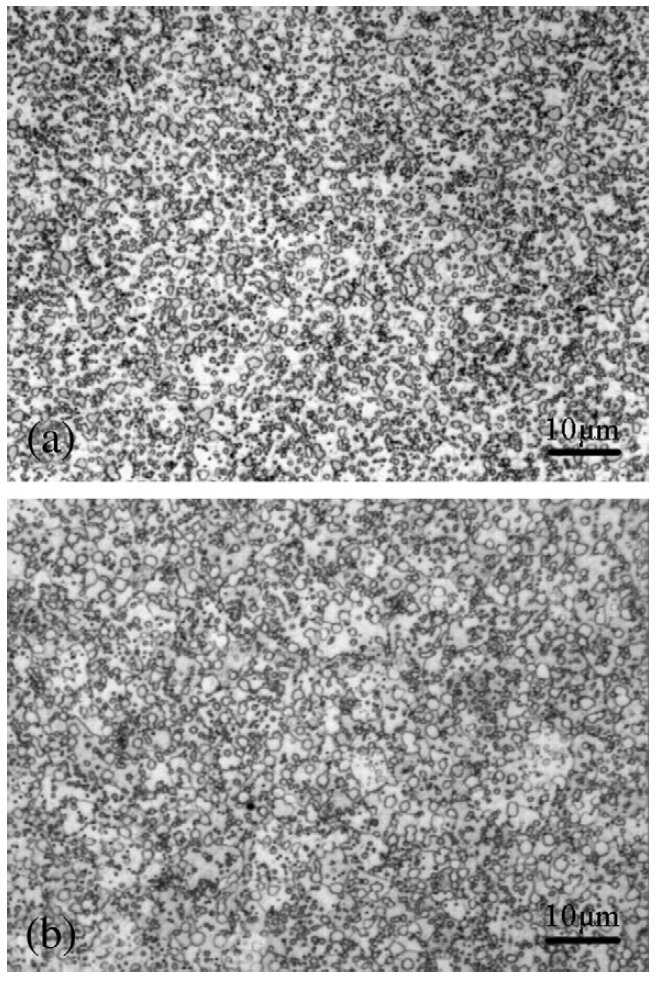
Figure 7: Comparison of V4 annealed microstructures: (a) spray forming, (b) powder metallurgy. Scale bars: 10 µm.
Carbide name: VC, M7C3
Record No.: 738
Carbide formula: VC, M7C3
Carbide type: MC, M7C3
Carbide composition in weight %: No data
Image type: LM, SEM, TEM
Steel name: Vanadis 4
Mat.No. (Wr.Nr.) designation: No data
DIN designation: No data
AISI/SAE/ASTM designation: No data
Other designation: No data
Steel group: PM high alloyed steels
Steel composition in weight %: 1.5% C, 1.0% Si, 0.4% Mn, 8.0% Cr, 1.5% Mo, 4.0% V.
Heat treatment/condition: The steels were melted in a vacuum induction furnace and
then cast into rods as feedstock for spray forming. The
feedstock was heated in an induction furnace and soaked at
above melting point for 20 min. The molten metal flow rate
was set at approximately 0.1 kg/s, using N2 with a pressure of
2.2 MPa as atomizing gas. The atomized droplets were cooled
and driven towards a revolving substrate to form a condensed
product. The distance from the nozzle to the substrate was set
as 360 mm, and the copper substrate rotated at a speed of
10 rpm during atomization and deposition. The whole spray
forming process was completed in about 40 s, and a gaussshaped
billet with about 130 mm in diameter and 30 mm in
height was obtained.
The as-sprayed billet was machined into specimens with a
thickness of 16 mm. The specimens were then heated at a rate
of 10°C/min to the rolling temperatures of 850°C, 900°C,
950°C, 1050°C, 1150°C, respectively. After holding for 15 min,
the specimens were rolled in a single 60% reduction pass, and
then cooled to room temperature in the sand. Three groups of
as-rolled specimens were annealed at 850°C, 900°C, 950°C
respectively, with each group containing five specimens rolled
at different temperatures. After being isothermal held for 2 h,
the specimens were cooled to 500°C at a rate of 30°C/h, and
then cooled in air. The most ideal microstructure, which was
obtained by the as-sprayed steel rolled at 1050°C and
annealed at 900°C, was chosen from the annealed specimens
and compared with that of the commercial V4 steel made by
powder metallurgy (By UDDEHOLM, Sweden).
Note: The as-sprayed microstructure of the Vanadis 4 steel was studied. It was observed that the
as-sprayed microstructure consists of martensite, retained austenite and uniformly
distributed spheroidal carbides. The Kurdiumov–Sachs crystallographic relation between
martensite and retained austenite is confirmed by transmission electron microscope. No
macro segregation or network carbides are found. This can be explained in terms of the
rapid solidification of spray forming for microstructure refining. The steel obtained by the
new processing has a uniform carbide distribution, and the average carbide size is even
smaller than the equivalent in the powder metallurgical Vanadis 4 steel. It was proved that
spray forming can be considered as a new and high-efficient way to replace powder
metallurgy for the production of Vanadis 4 steel.
As shown in Fig. 1a, the microstructure of the as-cast V4 steel
contains coarse eutectic carbides rich in Mo, Cr and V, which
segregate at the prior austenite grain boundaries during
solidification. These carbide arrangements will lead to very
low toughness and ductility of the materials. Fig. 1b shows
the microstructure of the as-sprayed material consisting of
fine, homogeneous and fully spheroidal grains ranging from
8 to 10 µm, which was substantially finer than the conventionally
cast equivalent. No carbide networks or large irregular
carbides were found, and the fine spheroidal carbides were
uniformly distributed throughout the microstructure. The
carbides located at the grain boundary were relatively larger
compared with those inside the grains.
The X-ray diffraction analysis revealed the presence of
martensite, and a considerable quantity of metastable
retained austenite in the matrix (Fig. 2). The cause of the
retained austenite, as reported for spray formed D2 and
rapid solidification D2 steel, is that much of the alloying elements were dissolved in the austenite during the spray forming and the rapid solidification stabilized the austenite
and therefore decreased the martensite start temperature Ms.
The TEM observation showed that the microstructure was
predominantly twined martensite. Some acicular martensite
and lath martensite were occasionally found. The representative
morphology of the martensite was shown in Fig. 3a
and b. It could be seen from Fig. 3c and d that the retained
austenite was arranged in form of layers between martensite strips. The selected area diffraction pattern showed the
Kurdiumov.Sachs orientation relation of (011)alpha'//(111)gamma between
the austenite and the martensite.
Carbides are important phases providing the steel with high
hardness and high wear resistance. The TEM analysis of the
cold work steel showed two types of carbides in the matrix:
vanadium riched MC (VC) and chromium riched M7C3 ((Cr,
Fe)7C3). It is interesting to compare the morphology of the
carbides in the as-sprayed microstructure with that in the ascast
one. As shown in Fig. 4a, many strip or short rod coarse
eutectic VC carbides (shown by arrows) are located along the
grain boundaries. But in the as-sprayed microstructure, fine
spheroidal VC carbides are uniformly distributed on the grain
boundaries, no coarse VC carbides could be found. The
morphology of VC is shown in Fig. 5a. This difference could
be explained by comparing the characteristics of VC formation
during solidification process. The vanadium is a strong
carbide-forming element, and the vanadium carbides
obtained by conventional cast could be divided into primary
carbides and eutectic carbides. The primary vanadium carbides
precipitate first from the molten steel during the
solidification process, and they grow without restriction
in various directions. Thus the primary carbides have a trend of forming large conglomerations. Other eutectic carbides are
in shape of strip or shot rod. This is because the VC is a typical
faceted crystal, but the austenite is non-faceted crystal,
therefore they tend to form divorced eutectic and the VC
carbides grow along the austenite grain boundaries during
solidification.
The uniform distribution of the spheroidal MC carbides in
the as-sprayed materials indicated that the eutectic reaction
was greatly restrained as the result of the rapid solidification.
The microstructural characteristics of the as-sprayed materials
critically depend upon the solidification history of the
droplets. During the spray forming process,
the melt stream is broken into finer droplets in diameters
ranging from 5 to 200 µm. The small droplets might have
solidified completely, the medium sized partially solidified
and the larger still in their liquidity when they arrived on the
deposition surface. The droplets will ideally form a mushy
zone consisted of liquid and solidified metal and the thickness
is only a few millimetres on the surface of the billet. The
mushy zone is expected to exist during the whole spray
forming process. When the droplets with high velocities hit
the surface of the mushy zone, dendrites form during flight
experience and semi solidified droplets may break up and
remelt. Those dendritic fragments having not been remelt will
be the new nuclei for the rapid solidification which is
promoted by the comparably cold atomizing gas. The formation
of spheroidal grains is proposed to evolve from the homogenization of dendrites without being deformed extensively,
or from the growth and coalescence of the deformed or
fractured dendrites.
The atomized droplets undergo a rapid solidification by the
effective convection of surrounding gas, and therefore, solute
segregation can be greatly depressed. In addition, the impact
of the droplets causing turbulences in the mushy zone leads to
further homogeneous thermal balance and chemical composition.
The amount of the residual liquid that could be at the
eutectic chemical composition is reduced due to the nonequilibrium
solidification process. Therefore the formation of
the elongated vanadium carbide is greatly suppressed and
results in fine spheroidal carbides.
The typical morphology of the VC carbide and its diffraction
patterns with zone axis of [011] are shown in Fig. 5. It is of
interest to note that the superlattice spots existed in the
diffraction pattern, which revealed the ultrafine structure of
the VC. The VC is in face-centered cubic (FCC) crystal
structure, and the atom diameter ratio of the carbon and the
vanadium is rC/rM=0.57(<0.59). The smaller the ratio the
higher the trend to form simple close-packed structure. The V
atoms are packed as face-centered cubic and the C atoms filled
in the octahedron interstice. But some C atoms may be absent
in the octahedron interstice of the crystal, which makes the
atomic ratio of C to V in the lattice less than 1. For example, the
chemical formula of vanadium carbide may vary form VC0.5 to VC.
Some eutectic M7C3 carbides existed along the grain
boundaries in the as-cast materials, and others precipitated
from the matrix and their morphology are shown in Fig. 4b,
which is region A in Fig. 4a. They were spherical and uniformly
distributed since they precipitated from the matrix. Most of
the M7C3 carbides in the as-sprayed microstructure are
spheroidal with diameter of about 180 nm, and most of them
are distributed inside the grains. Few dog-bone-like M7C3
carbides, as shown in Fig. 6, are observed. However, they are
only about 3–4 ìm long and are far smaller than those in the
as-cast microstructure.
After spray forming, the billets were hot rolled and then
annealed at different conditions in order to optimize the processing
parameters. The best microstructure obtained, Fig. 7a,
consists of fine carbides dispersed homogeneously in the
ferrite matrix. It was obtained by the as-sprayed steel rolled at
1050°C and then annealed at 900°C. The average carbide size
is even smaller than the equivalent in the powder metallurgical
V4 steel (Fig. 7b). The crucial reason for obtaining the
excellent microstructure is attributed to the spray forming
used for microstructure refinement. It is important to note
that, unlike conventional cast high alloy steels, the as-sprayed
steels showed good workability at temperatures ranging from
850 to 1150°C as no crack have been found in the specimens,
which have experienced severe deformation. The enhanced
workability of the as-sprayed steel is also attributed to the
refined microstructure with small equiaxed grains and evenly
distributed fine spheroidal carbides. The large deformation is usually used on conventional cast high alloy steels in order to
break up the coarse carbides, and the steels are sensitive to
crack during hot working because of the coarse grains and
coarse carbide networks.
Links: No data
Reference: Not shown in this demo version.