
Alphabetical Index
Chemical Composition of Steels
Keyword Search
Steel Names
Alloyed Steels
Carbon Steels
Cast Irons
Chromium Steels
Cold Work Tool Steels
Creep Resistant Steels
Hot Work Tool Steels
Molybdenum Steels
PM steels
Stainless Steels
Structural Steels
Tool Steels
Vanadium Steels
White Cast Irons
M2C Carbides
M3C Carbides
M7C3 Carbides
M23C6 Carbides
MC Carbides
Light Microscopy
EDS/WDS Microanalysis
Scanning Electron Microscopy
Transmission Electron Microscopy
X-Ray Diffraction
Help
Contact Us
Home
Carbides in tool steels with high chromium content
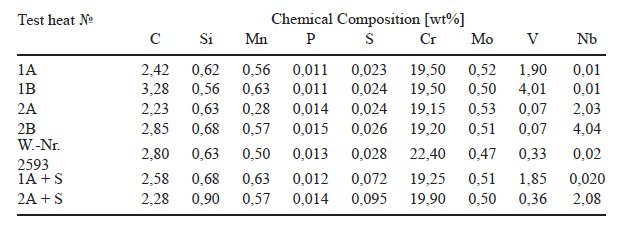
Table 1: Chemical composition of chromium alloyed castings with additions of vanadium
or niobium.

Figure 1: Microstructure of the cast alloy W.-Nr. 1.2593 (2.8% C; 25% Cr). Scale bar: 200, 20 µm.
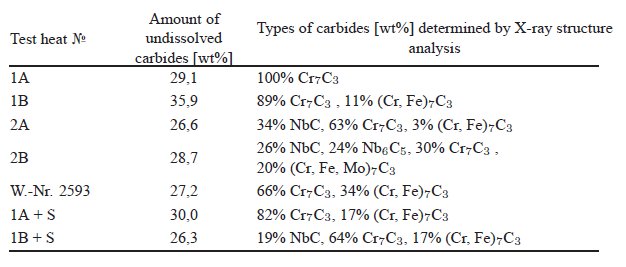
Table 2: Carbides in the tested cast alloys in the annealed condition.
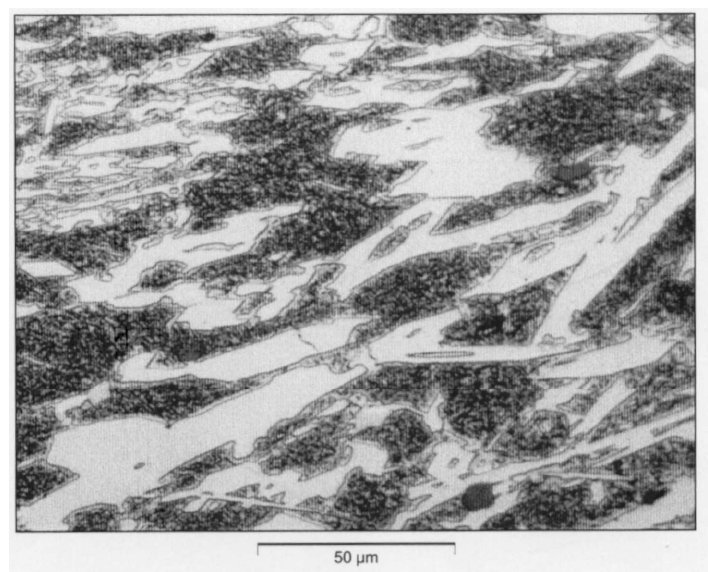
Figure 2: Microstructure of the cast alloy 1B (3.3% C; 19.5% Cr; 2.0% V). Scale bar: 50 µm.
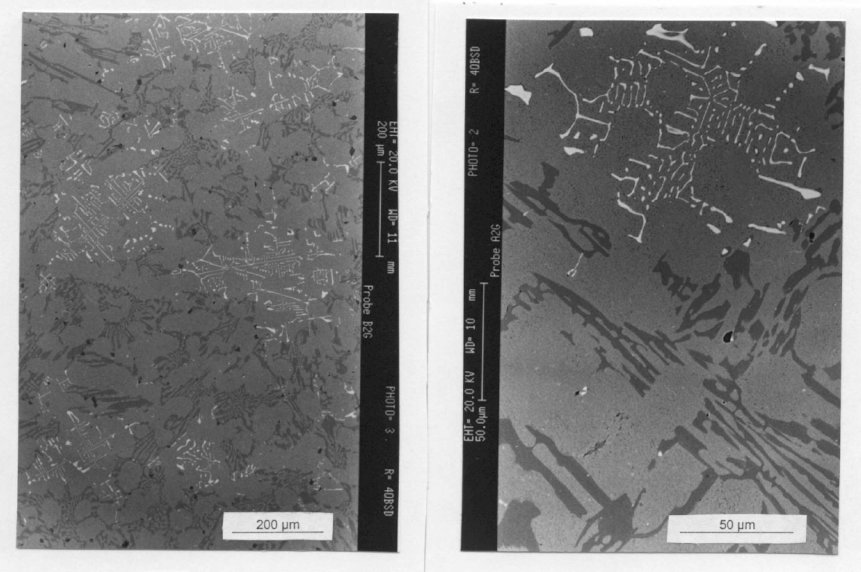
Figure 3: Microstructure of the cast alloy 2A (2.3% C; 19.2% Cr; 2.0% Nb). Bright
carbides NbC, dark carbides Cr7C3 (SEM photo). Scale bars: 200, 50 µm.
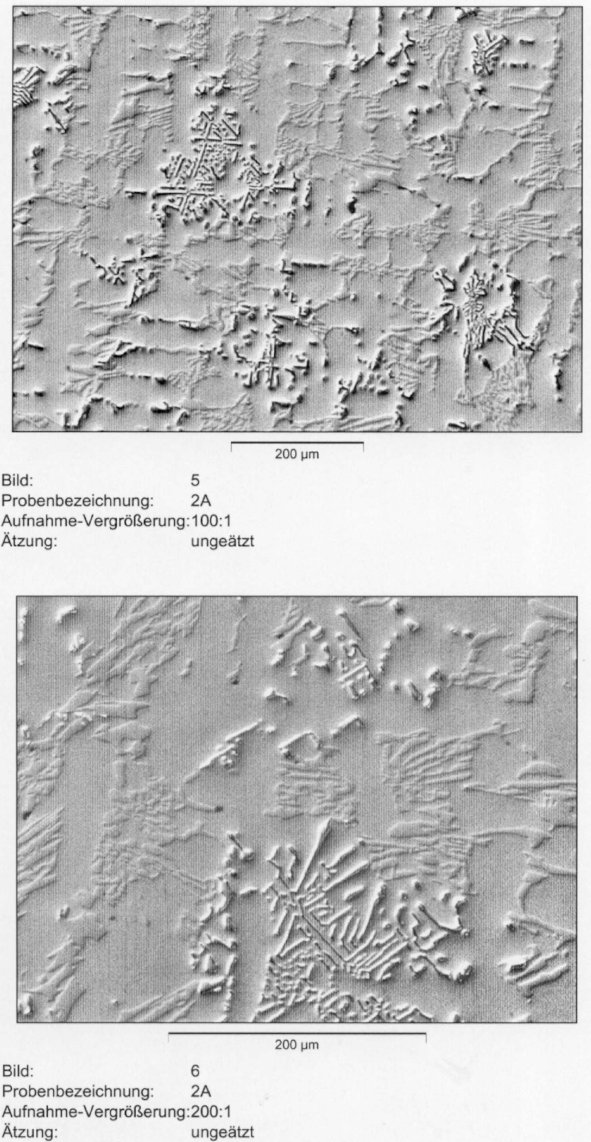
Figure 4: Microstructure of the cast alloy 2A (2.3 % C; 19.2 % Cr; 2.0 % Nb). Etched 5
% Nital and relief polished with alumina. Scale bar: 200 µm.
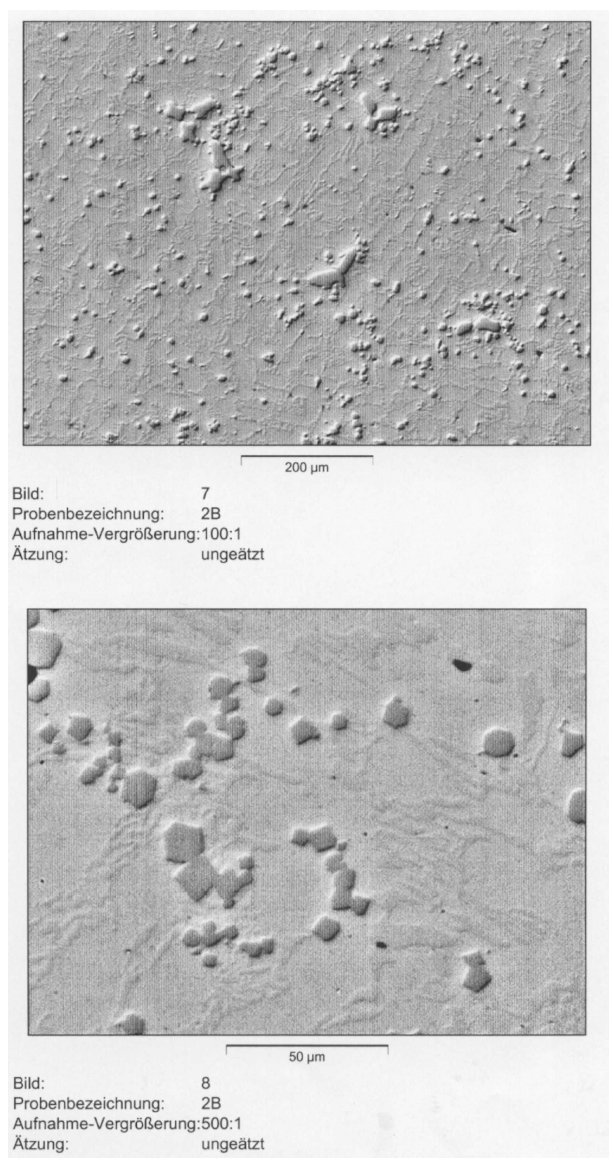
Figure 5: Microstructure of the cast alloy 2B (2.9% C; 19.2% Cr; 4.0% Nb). Etched 5%
Nital and relief polished with alumina. Scale bars: 200, 50 µm.
Carbide name: See the table 2.
Record No.: 744
Carbide formula: See the table 2.
Carbide type: See the table 2.
Carbide composition in weight %: No data
Image type: LM, SEM, XRD
Steel name: See the table 1.
Mat.No. (Wr.Nr.) designation: No data
DIN designation: No data
AISI/SAE/ASTM designation: No data
Other designation: No data
Steel group: Tool steels
Steel composition in weight %: See the table 1.
Heat treatment/condition: See the text bellow.
Note: There is a common wish to increase the wear resistance of cold work steel.
The possibility to improve wear resistance by the addition of more alloying
elements is limited, due to the loss of toughness. Therefore one tries to extend
the weight percentage of alloying elements like niobium and vanadium which
form very hard carbides.
The solubility of alloy carbides at austenitizing temperature and their
precipitation at higher tempering temperatures causes secondary hardness.
Contrary to vanadium carbides, niobium carbides have a very poor solubility
at hardening temperature.
Table 1 shows the analysis of these test melts with about
20% Chrome and additions of 2 and 4 wt% vanadium or niobium. The test
melts had a weight of 5 kg and were poured in ceramic moulds of 60mm
square. To reduce the cooling rate of the melts, the moulds were preheated
at 1000 C and embedded in sand.
Figure 1 shows the microstructure of a V- and Nb-free cast alloy W.-Nr.
2593 taken from an extrusion cylinder. All carbides in the microstructure of
Fig. 2 are of the type Cr7C3 as has been proven by X-ray structure analysis
(see Table 2).
By the addition of 2 or 4% vanadium, the carbide type in themicrostructure
remains unchanged Cr7C3 (Fig. 2). Vanadium carbides of the typeMC have
not been detected (see Table 2). It is to assume that the added vanadium is
completely dissolved in chromium carbide.
On the other hand the addition of niobium changes the microstructure
completely. After alloying niobium the structure shows two types of carbides,
the chromium rich carbide Cr7C3 and the niobium carbide NbC (see Table 2). In the alloy with about 2% Nb (alloy 2A) the niobium carbide has
an entectic appearance, but a crystallographic solidification of these niobium
carbides can be recognized in Fig. 3. These NbC must have been formed
between the begin of solidification and the eutectic temperature where the
residual melt decomposes to chromium carbide and austenite. Figure 4
shows the same microstructure etched and relief polished. One can clearly distinguish the two carbide types. The hard NbC is distinctly elevated while the softer Cr7C3 is more blurred in the background. In the melt with 4% niobium, the niobium carbide has another shape. At
this content niobium carbide directly precipitates at higher temperatures in the melt before the ferritic solidification of the alloy starts. By this way the NbC could here develop en exact crystallographic cubic or octaeder form as Fig. 5 shows. The cubic niobium carbides are random distributed in the structure. Some carbides have grown up to about 50 µm,(Fig. 5).
Links: No data
Reference: Not shown in this demo version.