
Alphabetical Index
Chemical Composition of Steels
Keyword Search
Steel Names
Alloyed Steels
Carbon Steels
Cast Irons
Chromium Steels
Cold Work Tool Steels
Creep Resistant Steels
Hot Work Tool Steels
Molybdenum Steels
PM steels
Stainless Steels
Structural Steels
Tool Steels
Vanadium Steels
White Cast Irons
M2C Carbides
M3C Carbides
M7C3 Carbides
M23C6 Carbides
MC Carbides
Light Microscopy
EDS/WDS Microanalysis
Scanning Electron Microscopy
Transmission Electron Microscopy
X-Ray Diffraction
Help
Contact Us
Home
Alloy Carbides
The addition to iron-carbon alloys of elements such as Ni, Si, Mn, which do
not form carbides in competition with cementite, does not basically alter the
microstructures formed after transformation. However, in the case of strong
carbide-forming elements such as Mo, Cr and W, cementite will be replaced by
the appropriate alloy carbides, often at relatively low alloying element
concentrations. Still stronger carbide- forming elements such as Nb, Ti and V are
capable of forming alloy carbides preferentially at alloying concentrations less
than 0.1 wt%. It would, therefore, be expected that the microstructures of steels
containing these elements would be radically altered.
Carbides are formed in steels only by iron and metals that stand to the left of
iron in the periodic table. The strong carbide-forming elements are shown in Fig. 1.
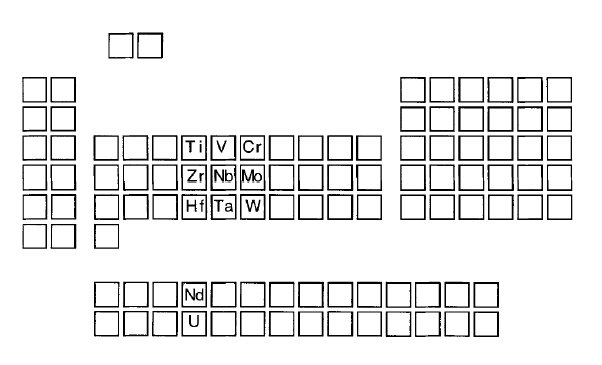
Figure 1: The periodic table showing the positions of strong carbide-forming elements.
In steel six kinds of carbides can be formed as shown in Table 1, where M denotes a sum of carbide-forming (metal) elements. The carbides placed in group I posses a complicated crystal structure; an examples is cementite (Fe3C), or Cr23C6. A specific structural feature of the carbides of group II as interstitial phases is a simple crystal lattice (e.g., TiC, WC, NbC and Mo2C).
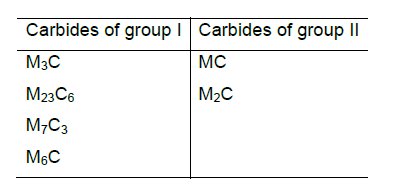
Table 1: Possible variants of carbide formation in steels.
The stability of the alloy carbides and nitrides in steels relative to that of cementite is shown in Fig. 2, where the enthalpies of formation (Hf) are plotted (cementite is the reference, i.e. Hf-cementite=0). It should be noted that the effectiveness of the carbides as strengtheners depends on the fineness of the dispersion and the volume fraction precipitated. The fineness of the dispersion depends on the activation energy barrier (G*) for nucleation which in turn is influenced by the free energy of formation of the carbide, the interfacial energy and the misfit. Fig. 2 can be used as a guide in this regard. The finest precipitate dispersions are generally obtained from VC, NbC, TiC, TaC and HfC. Theseare all close-packed intermetallic compounds. On the other hand the carbides with complex crystal structures and low heats of formation, e.g. M7C3, M6C and M23C6, generally form relatively coarse dispersions.
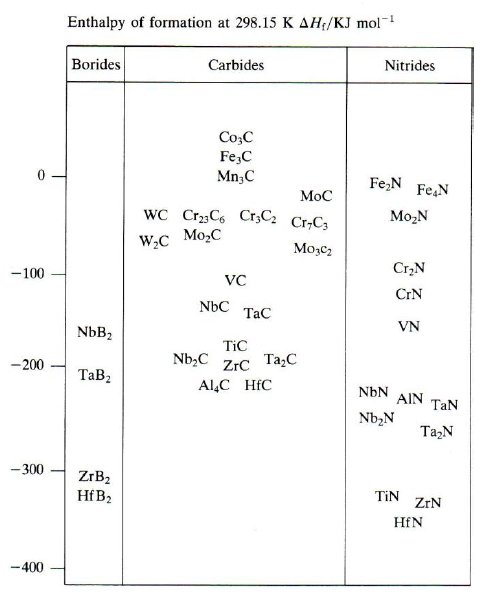
Figure 2: Enthalpies of formation of carbides, nitrides and borides.
The volume fraction of carbide precipitated depends on the solubility of the alloy carbide in the austenite prior to quenching, relative to the solubility in ferrite. Fig. 3 shows the solubility products of various carbides and nitrides in austenite as a function of temperature. The solubilities of these compounds in ferrite are very much lower and to a first approximation can be considered to be approximately equal. It is clear therefore that chromium, molybdenum and vanadium with highest solubilities in austenite, should precipitate in the highest volume fractions in the ferrite.
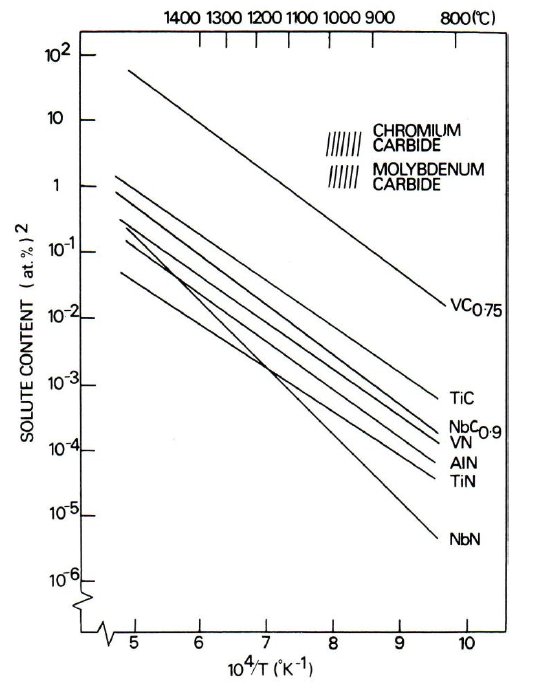
Figure 3: Solubility product of carbides and nitrides in austenite as a function of temperature.
Reference: Mehran Maalekian, The Effects of Alloying Elements on Steels (I), Technische Universität Graz, Institut für Werkstoffkunde, Schweißtechnik und Spanlose Formgebungsverfahren, Christian Doppler Laboratory for Early Stages of Precipitation, October 2007, pp. 10-13.